Two-ways validation of the feasibility of AI-based synthetic CT for MR-only brain radiotherapy
PD-0320
Abstract
Two-ways validation of the feasibility of AI-based synthetic CT for MR-only brain radiotherapy
Authors: Siti Masitho1, Juliane Szkitsak1, Johanna Grigo1, Florian Putz1, Rainer Fietkau1, Christoph Bert1
1University Hospital Erlangen, Radiation Oncology, Erlangen, Germany
Show Affiliations
Hide Affiliations
Purpose or Objective
Recent advances in MRI techniques
enable to perform the RT workflow entirely with only MR-imaging. For this
MR-only workflow, certain MRI images can be converted to a synthetic CT (sCT). sCTs
should provide similar HU values as planning CTs for dose calculation and sufficient
DRRs for daily positioning. A new artificial intelligence (AI)-based sCT
generation technique based on a single T1-VIBE DIXON sequence was evaluated
w.r.t. dose calculation and daily positioning for the brain radiotherapy
workflow.
Material and Methods
The T1-VIBE DIXON (in-phase and
opp-phase, 1.5x1.5x1.5mm3) was acquired at the 1.5 T MRI (Magnetom Sola,
Siemens Healthineers) for eight brain RT patients with various indications (including
metastasis, glioma, and whole brain). Patients were scanned in RT setup, which
utilizes an RT mask system, an RT flat table top, and a dedicated coil setup. Syngo.via
VB60 (Siemens Healthineers) was used to convert the MRI images to sCTs. For each patient, an RT plan
was created on the planning CT, which was then recalculated on the sCT (referred
to as TPCT→sCT);
and a second plan was created on the sCT, which was then recalculated on the
planning CT (TPsCT→CT).
To create comparable RT plans;
the RT mask system, CT markers, and table were previously removed from the planning
CT image. Mean absolute percentage deviation of D50 (∆D50[%])
and D0.01ccm (∆D0.01[%]) were evaluated for the target volumes
and OARs, respectively. Using Exactrac v6.0.6 (Brainlab), DRRs from the sCT were
fused with X-ray images recorded as part of the patient treatment. The difference
between the calculated couch shift/rotation after fusion and the recorded couch
shift using the planning CT was evaluated.
Results
∆D50[%] for PTV(TPCT→sCT)=0.28±0.27%
and for PTV(TPsCT→CT)=0.25±0.25%
were found (Fig.1). Meanwhile, ∆D50[%] for GTV(TPCT→sCT)=0.20±0.24%
and for GTV(TPsCT→CT)
=0.21±0.31%
were obtained. Largest mean ∆D0.01[%]
of OARs was found on the cochlea(L)(TPCT→sCT)=1.87±1.7% (max. ∆D0.01[%]=4.34%) and
the optical nerve(R)(TPsCT→CT)=0.83±0.73%.
(max. ∆D0.01[%]=2.17%). The overall mean ∆D0.01[%] of all OARs is 0.78±1.03%
for TPCT→sCT
and 0.43±0.49% for TPsCT→CT.
For DRR – X-ray fusion, median of couch shifts difference in lat./long./vert.
direction are -0.22±1.43mm/-0.56±2.68mm/-0.53±1.78mm and median of rotation
difference in roll/yaw/pitch direction are 0.17±1.0°/0.07±0.49°/-0.29±0.95°.
Overall, the mean ∆D50[%] and mean ∆D0.01[%] are <2%
for PTV, GTV, and various OARs, and the median of shift/rotation difference
during positioning are <1mm/1°.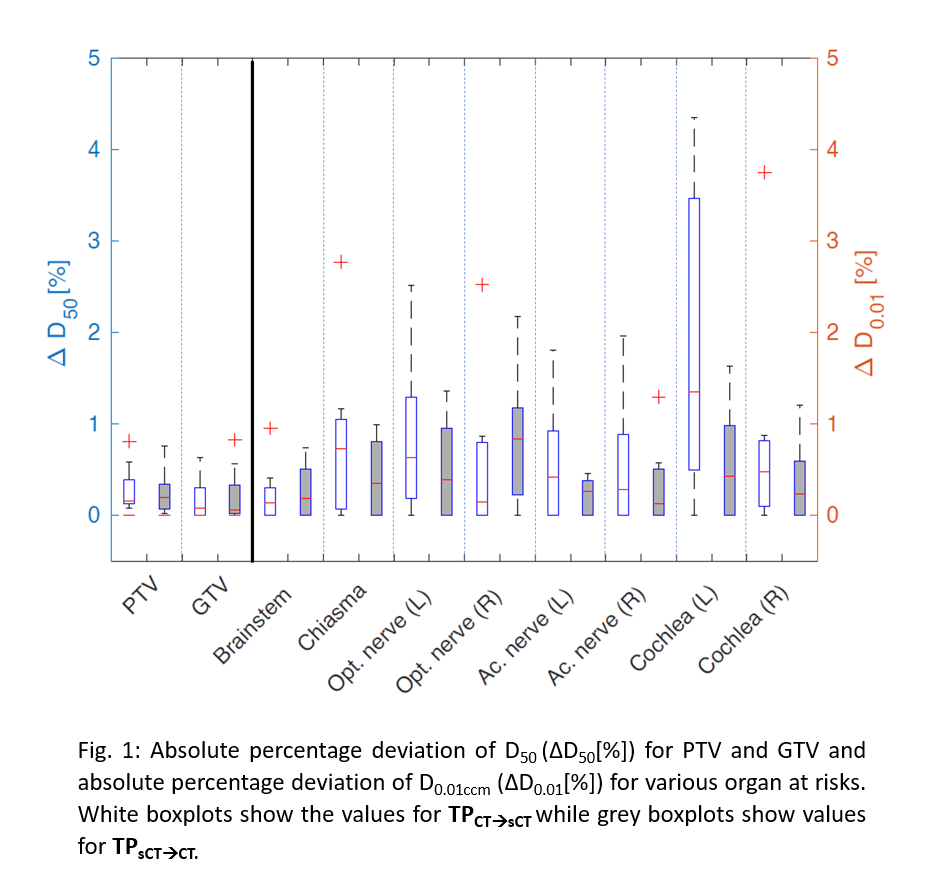
Conclusion
The AI-based sCT seems to be comparable with the planning CT for dose
calculation and daily positioning with stereoscopic X-ray imaging. The study is
currently still ongoing, in which more sCT plans will be evaluated.