SBRT of lung and liver oligometastases from breast cancer:a prospective non-randomized phase 2 trial
PD-0964
Abstract
SBRT of lung and liver oligometastases from breast cancer:a prospective non-randomized phase 2 trial
Authors: Beatrice Marini1,2, Luciana Di Cristina1,2, Veronica Vernier1,2, Ruggero Spoto1, Luca Dominici1, Francesca Lobefalo3, Lucia Paganini1, Ciro Franzese1,4, Davide Franceschini1, Marta Scorsetti1,4
1IRCCS Humanitas Research Hospital, Radiotherapy and Radiosurgery, Rozzano, Milan, Italy; 2Humanitas University, Department of Biomedical Sciences, Pieve Emanuele, Milan, Italy; 3IRCCS Humanitas Research Hospital, Radiotherapy and Radiosurgery, Rozzano, Milan, Italy; 4Humanitas Univesity, Department of Biomedical Sciences, Pieve Emanuele, Milan, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
We report mature toxicity and preliminary efficacy data from a phase II non randomized trial assessing the use of SBRT for lung and liver oligometastases from breast cancer. The co-primary endpoints were local control (LC) and both acute and late toxicity rates. Secondary endpoints were represented by distant progression free survival (DPFS), overall survival (OS), polyprogression free survival (PoFS), and time to start or change systemic therapy.
Material and Methods
Oligometastatic patients from breast cancer were treated with SBRT for up to 5 lung and/or liver lesions. Inclusion criteria were: age >18 years, ECOG 0-2, absence of life-threatening conditions, diagnosis of breast cancer, less than 5 lung/liver lesions (with a maximum diameter <5 cm), metastatic disease confined to the lungs and liver or extrapulmonary or extrahepatic disease stable or responding to systemic therapy, chemotherapy completed at least 3 weeks before treatment or started at least 2 weeks after RT, written informed consent. Different dose-fractionation schedules were used. 4D-CT scan and FDG-CT PET were acquired for simulation and fused for target definition.
Results
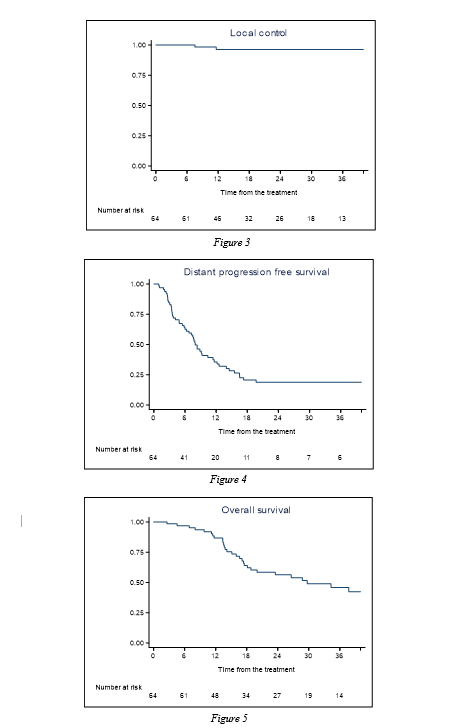
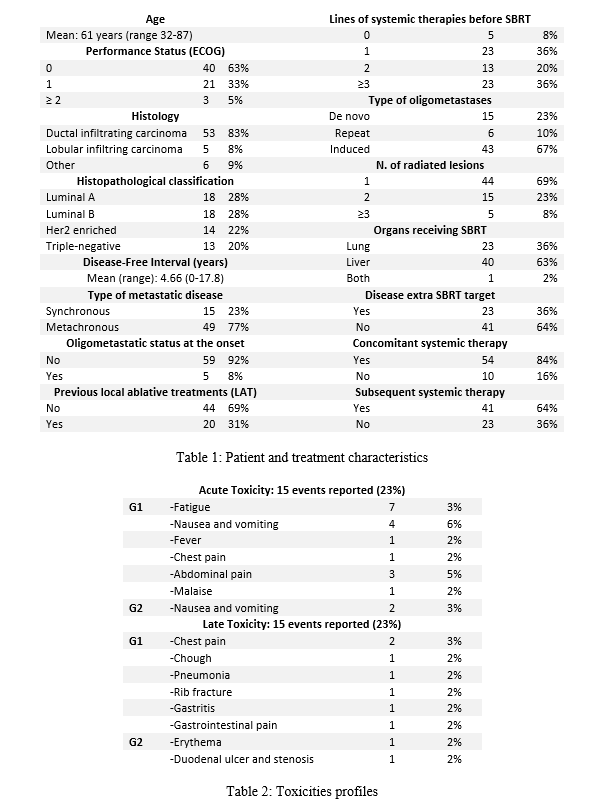
From 2015 to 2021, 64 patients for a total of 90 lesions were irradiated. Main patients and treatment characteristics are shown in table 1. Treatment was well tolerated, with no G3-4 toxicities. Acute and late toxicities are reported in table 2. Median follow-up was 19.4 months (range 2.6 – 73.1). LC rates were 96.2% at 1 and 3 years. Complete response, partial response and stable disease were detected in 39 (61%), 19 (30%), 6 (9%) patients, respectively. Median OS was 29.7 months. OS rates at 1 and 3 years were 86.5% and 45.8%. Median DPFS was 7.96 months, with a DPFS rate at 1 and 3 years of 35.5% and 18.77%. Median PoFS was 14.5 months, with a PoFS rate at 1 and 3 years of 64% and 23.5%. The median time to next systemic therapy was 7.9 months, with a 1-year rate of 30% and a 3-years rate of 2.5%. Kaplan-Meier curves for local control, distant progression free survival and overall survival are represented in Figure 3 through 5. In univariate analysis, the presence of extra-target lesions demonstrated a significant impact on OS, DPFS, and PoFS. Hormone-sensitive primary tumors were associated with a longer time to systemic therapy.
Conclusion
SBRT is an effective and safe treatment option for lung and liver oligometastatic breast cancer patients, reaching an excellent LC with limited toxicity, with promising rates of delay in disease progression.