Artificial-Intelligence based high precision Brachytherapy dose calculation
OC-0294
Abstract
Artificial-Intelligence based high precision Brachytherapy dose calculation
Authors: Sebastien Quetin1,2,3, Farhad Maleki4, Shirin Abbasinejad Enger2,3,5
1McGIll University, Biological and Biomedical Engineering, Montreal, Canada; 2Montreal Institute for Learning Algorithms, MILA, Montreal, Canada; 3Lady Davis Institute for Medical Research, Radiation Oncology, Montreal, Canada; 4University of Calgary, Computer Science, Calgary, Canada; 5McGill University, Medical Physics Unit, Department of Oncology, Montreal, Canada
Show Affiliations
Hide Affiliations
Purpose or Objective
RapidBrachyDL, a rapid and accurate radiation dose prediction model for high dose rate (HDR) brachytherapy applications via deep learning (DL) was previously developed by our group. The model was trained, validated and tested on prostate cases. The predicted doses with RapidBrachyDL showed an excellent agreement with ground truth MC simulations and also demonstrated good generalization to cervical data. However, dose calculations were performed on a coarse dose scoring grid (3*3*3 mm3 ). In this study, the model was further improved to predict dose in a finer voxel size (voxel 1*1*1 mm3 ) and provide a toolkit enabling patient specific high resolution dose calculations in HDR brachytherapy.
Material and Methods
In this retrospective study, CT images of breast cancer patients who received HDR brachytherapy treatment were used. RapidBrachyMCTPS, an open source Monte Carlo (MC) based treatment planning software was used to generate 3D dose maps, which were used as inputs for the DL model training. Two sets of simulations with 10^7 Iridium-192 decays were performed for every single dwell position; the absorbed dose was calculated in a detailed heterogeneous patient geometry as well as in a homogeneous water environment. The voxel was 1*1*1 mm3. Each MC dose map took on average 15 minutes to simulate on 64 CPU core nodes. For the DL training, the obtained dose maps and patient geometries were cropped with a novel technique such that only the part of the organs at risk relevant to the dosimetric indice computations were considered. That allowed for easier training of DL models on large data (3D volumes of large treatment sites + high resolution scoring grid). The subsequent water dose maps were fed into a “combining Unet” model along with the patient geometries to predict the heterogeneous dose maps. The architecture of the model was designed to handle the training on high resolution volumes while focusing on the necessary combination of features between the heterogeneous patient geometry and homogenous water dose. Data set from 70 breast cancer patients were used for the training of the model, 14 for validation and 14 for the test.
Results
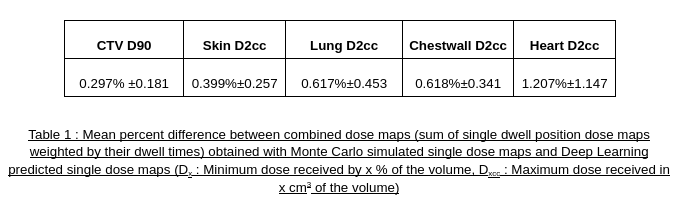
Training took 33 days on a A100 GPU. Once trained, the performance of the model was tested on 14 patients never seen by the model. On average, the test patients had 147 dwell positions per patient and the model predicted dwell position dose maps in 0.14 second. This represents dose maps for the entire plans predicted in 20 seconds. As shown in table 1, the proposed 3D DL regression demonstrated excellent agreement with the MC ground truth regarding dosimetric indices comparison.
Conclusion
Problems imposed by large treatment sites and high resolution were solved with a novel cropping strategy and DL model design. DL based dose predictions increase the precision of patient specific radiation treatments while decreasing the computation times to seconds, which will allow for implementation of model based dose calculations in a clinical workflow.