In total, 105 patients were evaluable for analysis with a median follow-up of 46 months. Among them, 44 patients (42%) experienced G4L at a median of 28 days after the start date of concurrent CRT. Figure 1 illustrates the absolute lymphocyte counts for the patients over the 5-week monitoring period during CRT stratified by treatment arm. The incidence of G4L for PBT versus IMRT was 27.3% versus 52.5%, respectively (relative risk 0.52, p=0.010). Induction chemotherapy (hazard ratio [HR] = 3.78, 95% confidence interval [CI] 1.59-8.97, p=0.003), baseline absolute lymphocyte count (ALC in K/μL; HR = 0.19, 95%CI 0.09-0.38, p<0.001), radiotherapy modality (IMRT vs. PBT; HR = 3.00, 95%CI 1.48-6.07, p=0.002), and planning treatment volume (PTV) (per 100 mL; HR = 1.13, 95%CI 1.01-1.27, p=0.033) were found to be significantly associated with G4L. Multivariable classification analysis partitioned patients into five subgroups for whom the incidence of G4L was observed in 0%, 14%, 35%, 70%, and 100% of patients (Figure 2). The benefit of PBT over IMRT was most pronounced in patients with an intermediate baseline ALC and large PTV (G4L in 35% versus 70%, respectively, p=0.011).
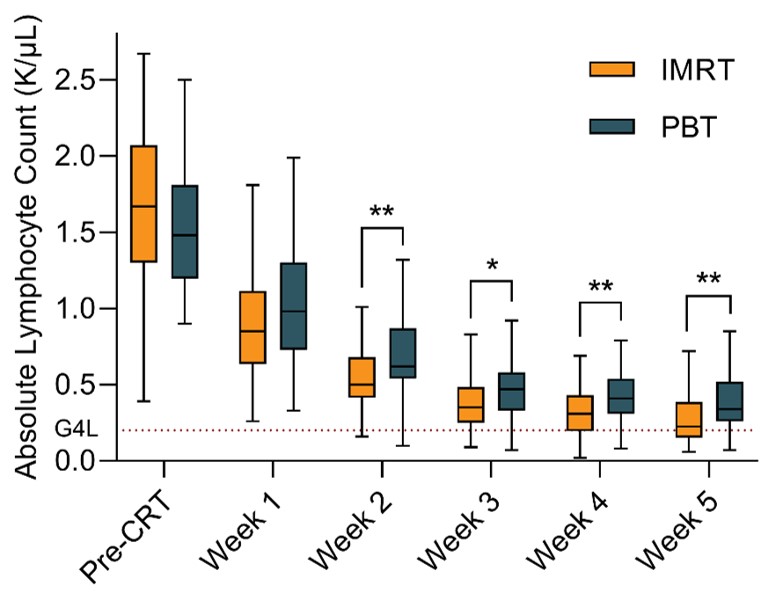
Figure 1. Distribution of absolute lymphocyte counts during concurrent CRT for patients stratified by treatment arm. G4L: grade 4 lymphopenia. *: p<0.05. **: p<0.01.
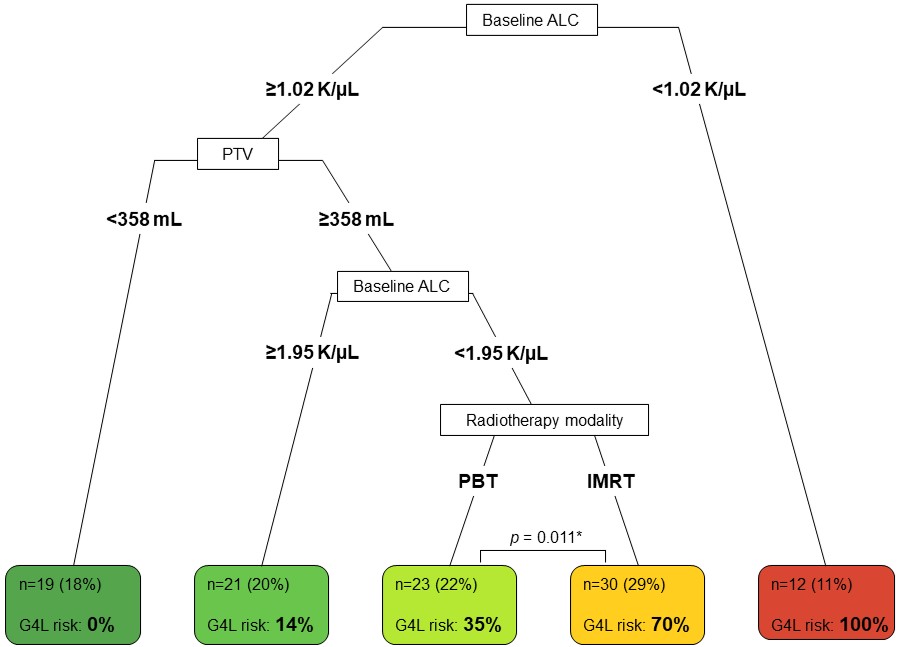
Figure 2. Decision risk tree model based on predictive factors for grade 4 lymphopenia (G4L).