Short course radiation improves pain from pancreatic cancer: A prospective phase II study (NTR5143)
OC-0100
Abstract
Short course radiation improves pain from pancreatic cancer: A prospective phase II study (NTR5143)
Authors: C. Paola Tello Valverde1, Gati Ebrahimi2, Johanna W. Wilmink3, Mirjam A. Sprangers4, Marc Jacobs4, Anna Bruynzeel5, Marc G.H. Besselink6, Hans Crezee7, Geertjan van Tienhoven5, Eva Versteijne5
1Amsterdam UMC, Radiation Oncology, Amsterdam , The Netherlands; 2Instituut Verbeeten, Radiation Oncology, Tilburg, The Netherlands; 3Amsterdam UMC, Medical Oncology, Amsterdam, The Netherlands; 4Amsterdam UMC, Medical Psychology, Amsterdam, The Netherlands; 5Amsterdam UMC, Radiation Oncology, Amsterdam, The Netherlands; 6Amsterdam UMC, Pancreatic and Hepatobiliary Surgery, Amsterdam, The Netherlands; 7Amsterdam UMC, Radiaton Oncology , Amsterdam, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
To assess the effect of short course palliative radiotherapy on pain severity, global quality of life (QoL), acute toxicity and overall survival (OS) in patients with pancreatic cancer-related pain.
Material and Methods
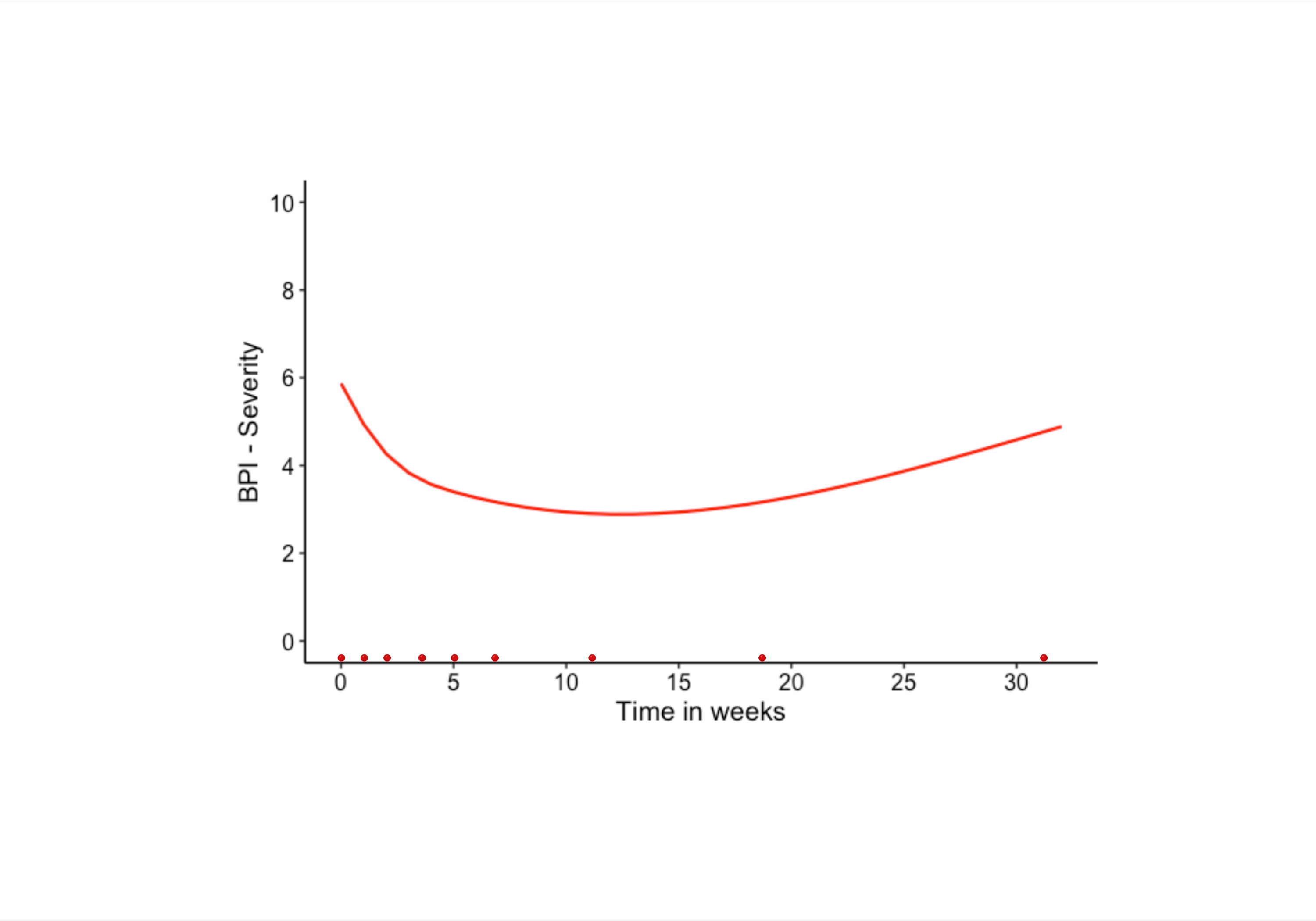
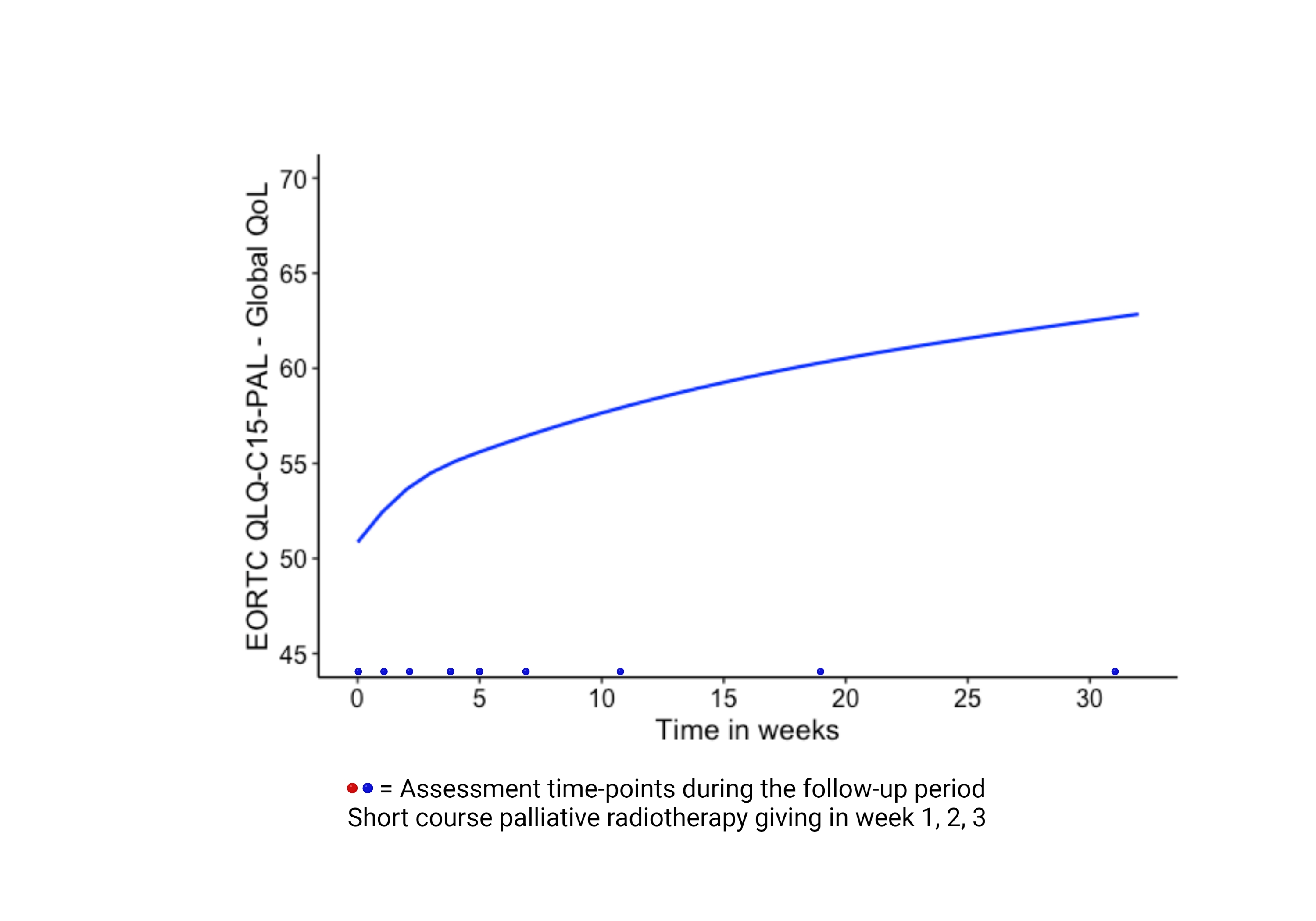
In this single-arm, prospective phase II study, after informed consent, 30 patients with moderate-to-severe pain of refractory pancreatic cancer were treated with short course palliative radiotherapy; 24Gy in three fractions, once a week, between 2015-2018. Primary endpoint was a change in pain severity with a clinically significant decrease of ≥2 points compared to baseline, measured using the Brief Pain Inventory questionnaire. Secondary endpoints were, change in global QoL measured using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-C15-PAL, acute toxicity based on clinician reporting and OS. Questionnaires were scheduled to be measured at nine time-points: at baseline (before the start of the radiotherapy), before the second and third radiotherapy fraction, and at four, five, seven, 11 and 19 weeks after the first radiotherapy fraction, and every three months thereafter if the patient was still alive. Patient-reported outcome measures were analyzed using joint modelling integrating a Cox regression and a mixed model.
Results
Overall, 29 patients received palliative radiotherapy. A total of 24 patients (80.0%) experienced pain relief, for whom 21 patients (70.0%) until death or last follow-up. Patients reported a significant mean pain severity reduction from 5.9 to 2.9 (p=0.017) during the first 11 weeks, followed by a slight increase to 3.4 (p=0.006) up to week 21 after the first radiotherapy fraction. Reduction of pain medication was seen in 16 patients (55.2%), pain medication remained unchanged in six patients (20.7%), and five (17.2%) required ≥25% increase of strong opioids. Global QoL significantly improved from 50.9 to 62.7 during the study period, p=<0.001. Joint model analysis showed better global QoL (p=0.039), decrease of nausea/vomiting (p=0.005) and lower insomnia symptoms (p=0.002) in patients treated with three (n=21) versus less than three (n=8) radiotherapy fractions. Grade 3 acute toxicity occurred in three patients, reporting transient flare-up combined with nausea and/or vomiting after the first fraction. No grade 4-5 acute toxicity was observed. Median OS was 11.8 weeks (range 0.9-238.8 weeks), with a 13.3% one-year actuarial OS rate.
Conclusion
This prospective phase II study of a short course palliative radiotherapy for pancreatic cancer-related pain showed a significant reduction of pain severity, for 70% of patients until death or last follow-up and an increase in global QoL over time, accompanied by mostly mild toxicities.