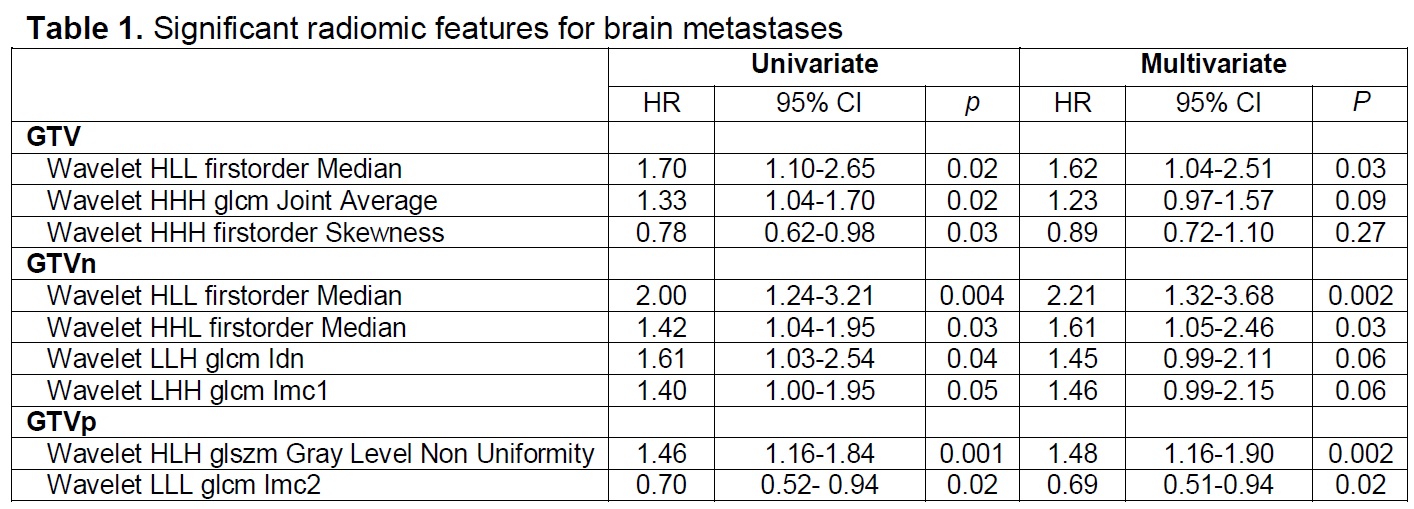
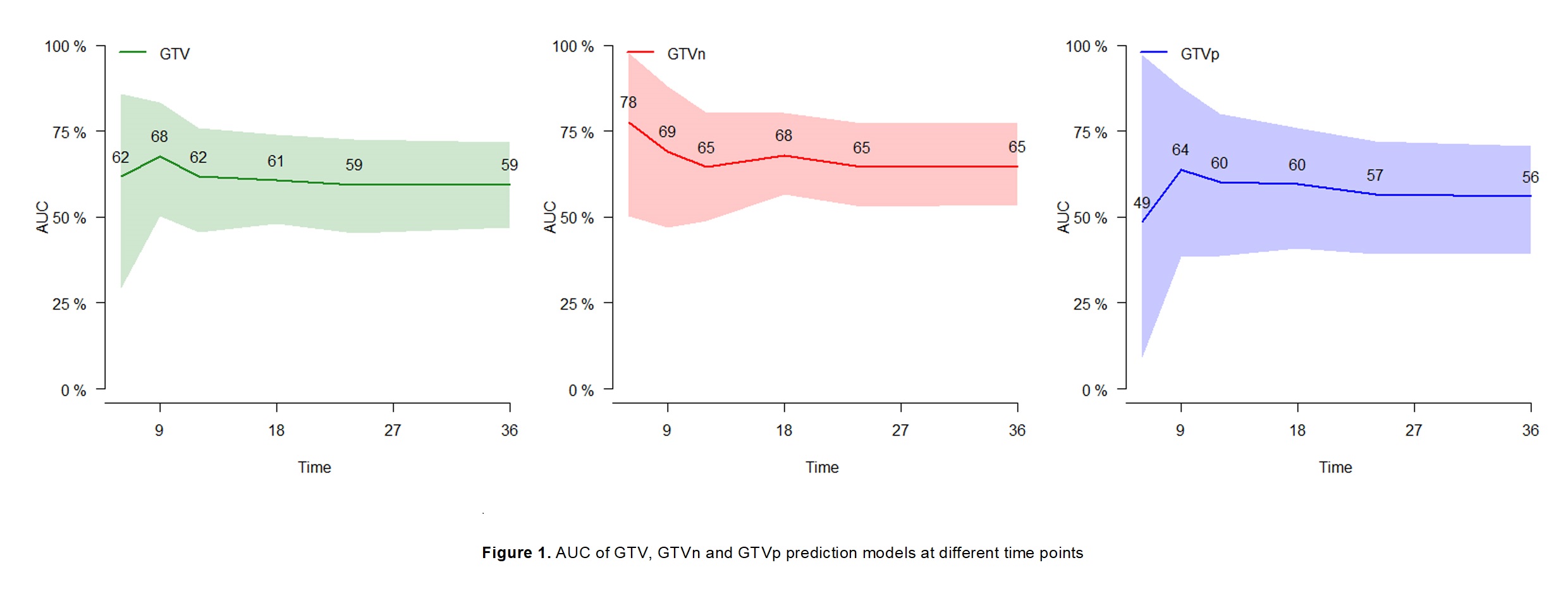
In total, 296 out of 497 patients were eligible, 157 (53%) were male, 112 (37.8%) were squamous cell,160 (54.1%) were stage IIIA, and 73 (24.7%) received immunotherapy. The median age was 66y (IQR 60-72). Within a median follow up of 55.3 months (95% CI 48.0-62.7 months), 180 (60.8%) patients died, 46 (15.5%) developed BM at a median time of 10.8 months. The median overall survival was 29.9 months (95%CI 23.1-36.7). GTV was available for all patients, of which 266 GTVn and 274 GTVp were distinguishable. In all, 861 features were extracted. Univariate analysis showed that 3 GTV, 4 GTVn, and 2 GTVp features were significantly associated with BM development (p<0.05). Multivariate models showed that Wavelet-HLL-first-order-Median (HR=1.62, 95% CI 1.04-2.51, p=0.03) was a significant GTV feature, Wavelet- HLL-first-order-Median (HR=2.21, 95% CI 1.32-3.68, p=0.002) and Wavelet-HHL-firstorder-Median (HR=1.61, 95% CI 1.05-2.46, p=0.03) were significant GTVn features, Wavelet-LLH-glcm-Idn (p=0.06) and Wavelet-LHH-glcm-Imc1 (p=0.06) were marginally significant GTVn features, Wavelet-HLH-glszm-Gray-Level-Non-Uniformity (HR=1.48, 95% CI: 1.16-1.90, p=0.002) and Wavelet-LLL-glcm-Imc2 (HR=0.69, 95% CI: 0.51-0.94,p=0.02) were significant GTVp features (Table). The AUC of the GTV, GTVn, GTVp model ranged from 0.59-0.68, 0.65-0.78, 0.49-0.64, respectively (Figure).