Deep learning NTCP model for late dysphagia based on 3D dose, CT and segmentations
Suzanne P.M. de Vette,
The Netherlands
OC-0088
Abstract
Deep learning NTCP model for late dysphagia based on 3D dose, CT and segmentations
Authors: Suzanne P.M. de Vette1, Hung Chu2, Hendrike Neh1, Roel J.H.M. Steenbakkers1, Peter M.A. van Ooijen1, Clifton D. Fuller3, Johannes A. Langendijk1, Nanna M. Sijtsema1, Lisanne V. van Dijk1
1University Medical Centre Groningen, Radiotherapy, Groningen, The Netherlands; 2University Medical Centre Groningen, Radiotherapy, Goningen, The Netherlands; 3MD Anderson Cancer Center, Radiation Oncology, Houston, USA
Show Affiliations
Hide Affiliations
Purpose or Objective
Dysphagia after radiotherapy for head and neck cancer (HNC) has a large impact on patient-reported quality of life. Normal tissue complication probability (NTCP) models that predict the dysphagia risk after radiotherapy can be utilized to guide therapy decisions. These NTCP models are currently based on discrete dose parameters of a distinct set of swallowing-related structures (e.g., the pharyngeal constrictor muscles (PCMs) and the oral cavity). However, dysphagia development is a complicated inter-connected process involving many swallowing muscles and structures. Deep learning (DL) has the potential to improve dysphagia prediction, as it can predict based on the whole 3D dose distribution instead of reducing this to a single dose value. The aim of this study is to improve the prediction of dysphagia at 6 months after radiotherapy with 3D DL models compared to conventional NTCP models.
Material and Methods
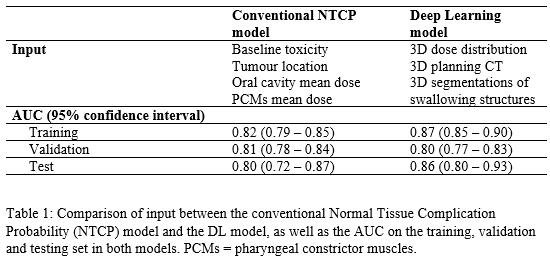
At a single centre, 1113 HNC patients were included that received primary radiotherapy between 2007 and 2021. The cohort was split in a training (70%), cross-validation (15%), and independent “never-seen-by-the-model” test (15%) set. The endpoint was grade ≥2 physician-rated dysphagia at 6 months after radiotherapy (CTCAEv4.0). Input for the DL model were 3D planning CTs, dose distributions and swallowing structure segmentations (Brouwer et al., 2015). After hyperparameter tuning, a ResNet Deep Convolutional Neural Network (DCNN) was trained and optimized on a combination of cross-entropy and Area Under the Curve (AUC).
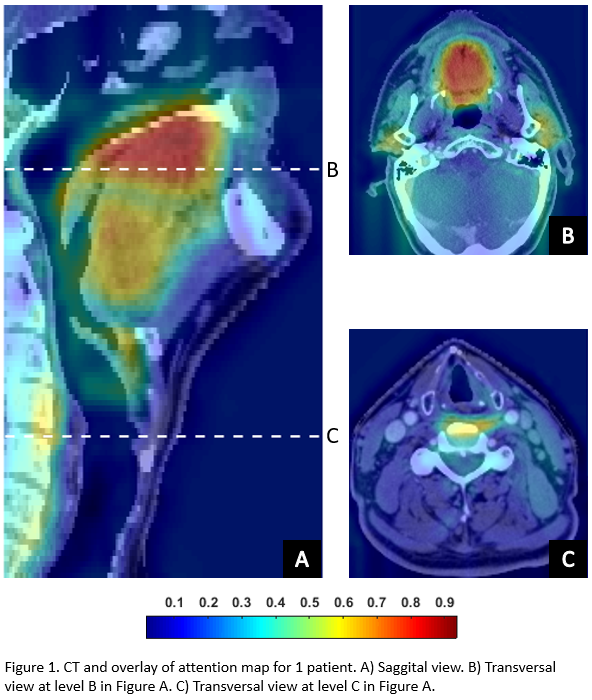
For comparison with the DL model, the performance of the recently published dysphagia NTCP model, which was trained on a comprehensive study cohort, was determined in our current dataset. Furthermore, attention maps that highlight the areas that the DL model focuses on per individual patient were visually evaluated.
Results
Hyperparameter tuning showed that a combination of the AdaBelief optimizer and a batch of 8 resulted in the highest training and validation AUC (Table 1). The final DL NTCP model showed excellent performance in the independent test set (AUC = 0.86 (95% Confidence Interval [0.80–0.93])). This performance was improved compared to the conventional NTCP model (AUC = 0.80 [0.72-0.87]), suggesting that 3D information enhanced dysphagia prediction. The attention maps revealed that the regions that have previously been associated with dysphagia development (e.g. the oral cavity and PCMs) were highlighted by the DL model (Figure 1), yet additionally the DL model focused on the parotid glands, submandibular glands, base-of-tongue and the larynx area.
Conclusion
Combining 3D information of the dose distribution, CT and swallowing-related segmentations in a DL model showed an improved prediction of late dysphagia compared to conventional NTCP models based on discrete dose parameters. Currently these models are in the process of being externally validated to evaluate their generalizability.