Long-term results of an SBRT dose-escalation trial for bone and lymph node metastases (NCT03486431)
OC-0267
Abstract
Long-term results of an SBRT dose-escalation trial for bone and lymph node metastases (NCT03486431)
Authors: Carole Mercier1,2, Charlotte Billiet1,2, Ines Joye1,2, Paul Meijnders1,2, Daan Nevens1,3, Piet Ost1,4, Dirk Verellen1,2, Piet Dirix1,2
1Iridium Netwerk, Radiation Oncology, Antwerp, Belgium; 2University Antwerp, Integrated Personalised and Precision Oncology Network, Antwerp, Belgium; 3University Antwerp, Integrated Personalised and Precision Oncology Network, Ghent, Belgium; 4Ghent University, Department of Human Structure and Repair, Antwerp, Belgium
Show Affiliations
Hide Affiliations
Purpose or Objective
To present long-term results of a stereotactic body radiotherapy (SBRT) trial for non-spine bone and lymph node oligometastases, comparing toxicity and efficacy of the three most used fractionation (i.e. 5, 3 or single fraction) schedules (NCT03486431).
Material and Methods
This was a prospective non-randomized trial including patients with 3 or less non-spine bone and/or lymph node metastases. In the first, second and third cohort of each 30 patients, all detected metastases were treated to 5 x 7 Gy, 3 x 10 Gy or 1 x 20 Gy, respectively. SBRT could be combined with standard of care systemic treatment. The primary endpoint was 6-month dose-limiting (grade ≥3) toxicity (DLT) rate (previously published) and secondary endpoints were late toxicity, local failure (LF) and progression-free survival (PFS). The study opened in July 2017 and closed in December 2018. In the current report, long-term follow-up was analyzed through September 2022.
Results
Ninety patients received SBRT to 101 metastases. Median age was 69 (IQR 62-75) and the majority of patients (74%) were male. The most common primary tumor types were prostate (n=52; 58%), breast (n=13; 14%) and lung (n=8; 9%). The three groups were well-balanced for patient and tumor characteristics. The primary endpoint was previously reported after a median follow-up of 17.2 months. At that time, no DLT was observed in any of the treatment arms.
Median follow-up for the current analysis was 50 months. Rates of grade 2 SBRT-related acute or late toxicity were 13%,7 % and 10% in cohort 1 through 3, respectively (p=0.9). No grade 3-5 toxicities were observed. LF occurred in 9% vs 3% vs 6% of lesions for the first, second and third cohort, respectively (p=0.5). Overall estimated 4-year local control rate was 93%. There was no difference in local control rates between schedules (Figure 1).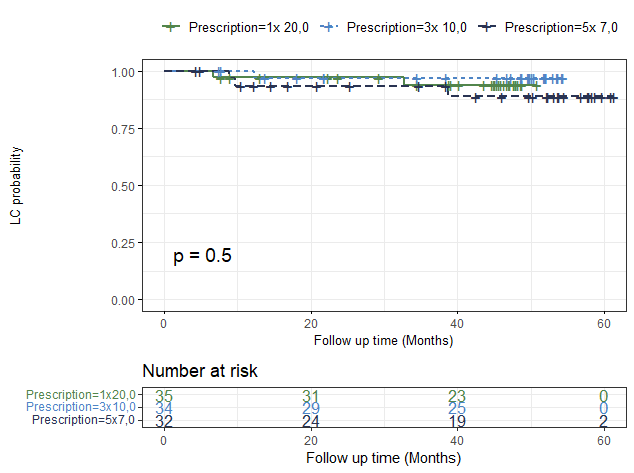
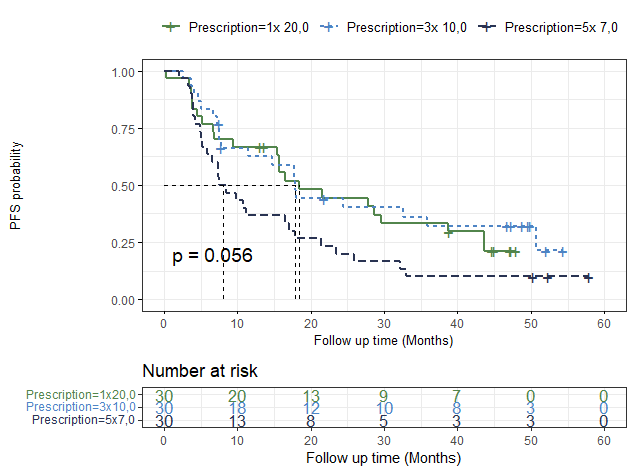
Median PFS was 16.5 months (95% CI 9.8 - 21.5), with a 4-year PFS of 21% (95% CI 14 - 32). Twenty-one patients (23%) remained long-term disease-free, 33/90 (37%) patients were oligoprogressive (≤3 new lesions) at first recurrence and 36/90 (40%) developed a polymetastatic relapse. Interestingly, median PFS was lower in the group treated with 5 fractions (8 months) compared to both other groups (18 months, p = 0.056) (Figure 2). Median OS was not reached (95% CI 53 months – NA) and 4-year OS was 68% (95% CI 59 – 78).
Conclusion
At long-term follow-up, no new safety signals were observed and SBRT remained entirely safe, whatever the fractionation. Also, local control remained excellent and did not differ between schedules. The overall PFS rate (21% at 4 years) was consistent with the literature. However, while neither intended nor powered for such an analysis, the results might suggest that 3 or single fraction schedules could lead to superior oncological outcomes. Of note, only 40% of patients went on to develop polymetastatic disease, confirming the existence of a true oligometastatic state.