LDR monotherapy versus LDR boost for intermediate-risk prostate cancer: long-term outcomes
OC-0625
Abstract
LDR monotherapy versus LDR boost for intermediate-risk prostate cancer: long-term outcomes
Authors: Alai Goñi Ramirez1, Belen De Paula1, Eva Saenz de Urturi1, Vicent Pastor Sanchis2, Albert Bartrés2, Mikel Egiguren1, María Pagola1, Daniel Alberto Roura1, Arancha Ayete1, Sara Palacios1, Usoa Iceta1, Leyre Gonzalez1, David Ignacio Ortiz de Urbina1, Veronica Alba2, Melanie Erzilbengoa2, Jesus Rosa Nieto1
1Onkologikoa - UGC Oncología Gipuzkoa, Radiation Oncology, San Sebastian, Spain; 2Onkologikoa - UGC Oncología Gipuzkoa, Medical Physics, San Sebastian, Spain
Show Affiliations
Hide Affiliations
Purpose or Objective
To determinate and compare long-term biochemical control rates, survival and toxicity outcomes between low-dose-rate (LDR) iodine-seed brachytherapy treatment modalities (iodine-seed monotherapy versus iodine-seed boost) in treatment of intermediate prostate cancer patients.
Material and Methods
Between January-2001and December-2017, 436 patients with intermediate prostate cancer were treated in our institution with combined 46Gy external beam radiotherapy plus 108Gy iodine-seed brachytherapy boost (BT-B) or with 144Gy iodine-seed brachytherapy monotherapy (BT-M). Pre-planned brachytherapy was used in both treatment modalities and use of androgen deprivation therapy (ADT) was allowed in the BT-B cohort. For this study, men with prostate cancer, clinical stage T1c-T2b and either Gleason Score (GS) 2-6 / PSA 10-20ng/mL or GS 7 / PSA <10ng/mL were eligible for the analysis. Those with less than 3-year follow-up and less than 3 post treatment prostate-specific antigen (PSA) were excluded. Clinical outcomes and toxicity were evaluated and compared. Toxicity was evaluated using Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Results
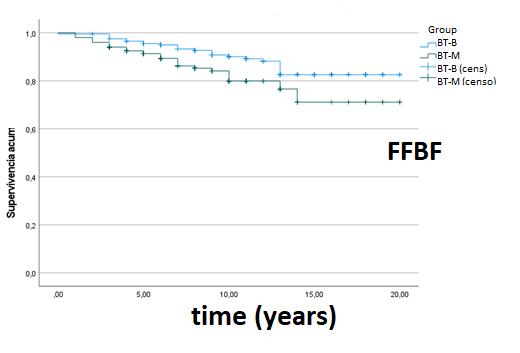
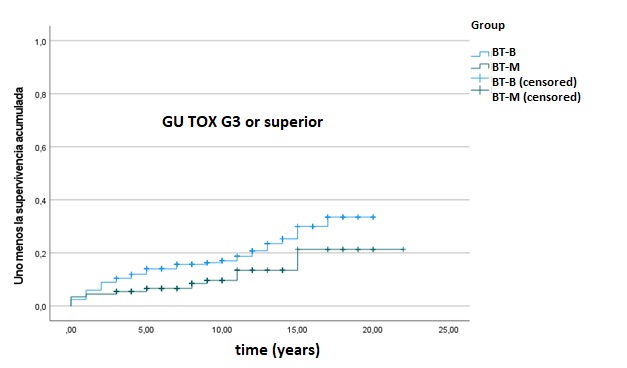
About 402 of 436 patients satisfied inclusion criteria (201 BT-M and 201 BT-B) and were included in the final analysis. The median follow-up was 10 years for the entire cohort. Median age was 72 years. Freedom from biochemical failure (FFBF) defined by Phoenix-criteria was 91.5% at 5 years, 85.6% at 10 years and 84.6% at 15 years in BT-M group, and 95.5%, 91% and 88% at 5, 10 and 15 years in BT-B group, respectively, statistically unfavorable for BT-M group. There was no difference in overall survival (OS) and metastases free survival (MFS) at 10 years between modalities; 73,6% and 95.5% in BT-M group and 76.6% and 96.5% in BT-B group, respectively. Genitourinary (GU) grade 3 or higher late toxicity was statistically unfavorable for BT-B: 13.4%, 16.4% and 20.9% at 5, 10 and 15 years in BT-B group, and 6.5%, 8% and 9.5% at 5, 10 and 15 years in BT-M group, respectively. No significant difference was found on gastrointestinal (GI) grade 3 or higher late toxicity: 2.5% at 5 years, 3% at 10 years and 3.5% at 15 years in BT-B group and 0.5% for both 5 and 10 years in BT-M group.
Conclusion
Among men with intermediate-risk prostate cancer, FFBF was statistically unfavorable for BT-M, although both BT-M and BT-B achieve excellent long-term biochemical and survival rates. On the other hand, long-follow up showed that late grade 3 GU toxicity was statistically unfavorable for the BT-B group. Late grade 3 or higher GI toxicity was limited in both groups, but there were fewer late effects in the BT-M group.