Late toxicity following stereotactic radiotherapy (SABR) for central and ultracentral lung tumors
Hilâl Tekatli,
The Netherlands
OC-0609
Abstract
Late toxicity following stereotactic radiotherapy (SABR) for central and ultracentral lung tumors
Authors: Hilâl Tekatli1, Frank Lagerwaard1, Rik van Eekelen2, Suresh Senan1
1Amsterdam UMC, Radiation Oncology, Amsterdam, The Netherlands; 2Amsterdam UMC, Department of Epidemiology and Data Science, Amsterdam, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
SABR for central and ultracentral lung tumors is associated with increased toxicity, including lung hemorrhage and airway obstruction. As there is limited data available on toxicities manifesting more than 2 years post-SABR, we studied toxicity in long-term survivors.
Material and Methods
Patients with primary or recurrent central NSCLC treated using VMAT between 2008 and 2015 were identified from our institutional database after institutional Ethics approval. Moderately central tumors generally received 60 Gy in 8 fractions, and when the PTV overlapped the trachea or main bronchi, 60 Gy was delivered in 12 fractions. No dose constraints for the heart, trachea, and bronchi were specified during this period. Toxicity was scored by at least 2 physicians using CTCAE v5, and was consensus-based. Bronchial airway toxicity was scored relative to pre-SABR and was defined as stricture, obstruction or atelectasis. Multivariable Cox proportional hazards regression models were used to estimate hazard ratios.
Results
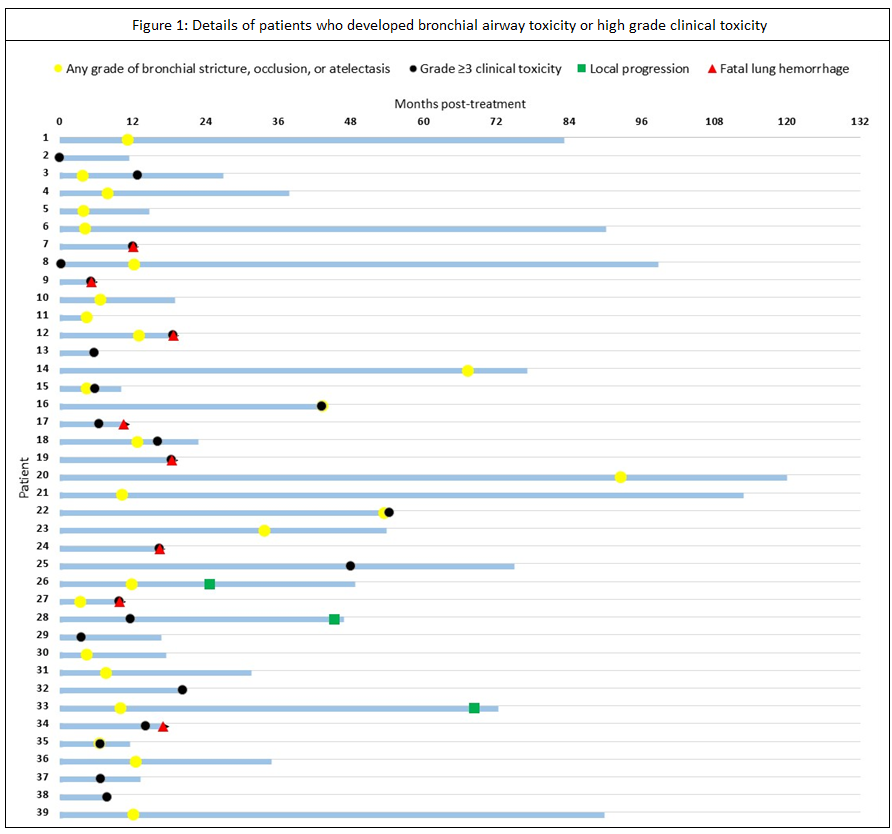
102 patients were identified. The majority (60%) were treated with 8 fractions. Tumor diameter exceeded 5 cm in 46%. PTV overlapped the trachea, main bronchus or intermediate bronchus in 48% of patients, the lobar bronchi in 21%, and segmental bronchi in 23%. A PTV located ≤2 cm from the proximal bronchial tree without overlap of central airways comprised 8% of the total. Median OS for the entire cohort was 28.7 months (95% CI 16.6-40.9) after a median follow-up for surviving patients of 138.2 months (95% CI 109.0-167.4). Local recurrences developed in 11%, regional recurrences in 7%, distant relapses in 15%. An isolated local recurrence was seen in only 4% of patients. Grade ≥3 clinical toxicity was observed in 23% of patients, with actuarial rates of 15% (95% CI 7-22) and 26% (95% CI 15-36) after 1 and 4 years, respectively (Figure 1). Possible or likely treatment-related fatal lung bleeding was observed in 8%. Any grade of bronchial toxicity was observed in 25%; and 76% developed toxicity in ≤12 months post-SABR. Bronchial atelectasis developed in 15% and was observed in ≤12 months (range 3.4-91.2) in 80%. Grade ≥3 clinical toxicity and/or bronchial obstruction/atelectasis were observed in 33% of patients, with actuarial rates at 1 and 4 years post-SABR being 24% (95% CI 15-32) and 40% (95% CI 28-50), respectively. Multivariable analysis revealed that high grade clinical toxicity and/or bronchial obstruction/atelectasis were significantly associated with PTV overlap with lobar bronchi (HR 20.8; 95% CI 2.7-162.9), PTV overlap with trachea/main bronchi/intermediate bronchi (HR 12.8; 95% CI 1.6-101.3), and endobronchial tumors (HR 2.8; 95 CI 1.3-6.3).
Conclusion
After central SABR, the rates of high grade clinical toxicity and bronchial obstruction/atelectasis almost doubled to 40% in patients surviving more than three years. This highlights the importance of identifying better planning constraints for central airways, and for improved SABR delivery approaches for central tumors.