A Delphi consensus study on SBRT for oligometastatic renal cell carcinoma (RCC) an ESTRO ACROP study
OC-0436
Abstract
A Delphi consensus study on SBRT for oligometastatic renal cell carcinoma (RCC) an ESTRO ACROP study
Authors: Giulia Marvaso1,2, Mattia Zaffaroni3, Shankar Siva4, David Pasquier5, Maria Giulia Vincini1, Giulia Corrao1, Barbara Alicja Jereczek-Fossa3,6, On behalf of the ESTRO ACROP group7
1IEO, European Institute of Oncology IRCCS, Division of Radiation Oncology, Milan, Italy; 2University of Milan, Department of Oncology and Hemato-Oncology, Milan, Italy; 3IEO, European Institute of Oncology IRCCS, Division of Radiation Oncology , Milan, Italy; 4Peter MacCallum Cancer Centre, University of Melbourne, Sir Peter MacCallum Department of Oncology, Melbourbe, Australia; 5Centre Oscar-Lambret, Service de radiothérapie oncologie, Lille, France; 6University of Milan, Department of Oncology and Hemato-Oncology , Milan, Italy; 7ESTRO ACROP, ESTRO ACROP, Milan, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
Currently, there are no guidelines on the management of oligometastatic RCC with SBRT. The purpose of this ESTRO ACROP project is to explore the expert opinion on the management of oligometastatic RCC patients using SBRT on extracranial metastases, with the aim of developing consensus recommendations to help with management decisions for treatment, improve patient selection, prescription doses and management alongside systemic therapy.
Material and Methods
A questionnaire on SBRT in oligometastatic RCC was prepared by a core group and reviewed by a panel of 10 prominent experts in the field. The final version of the questionnaire included 58 questions dealing with controversial issues related to oligometastatic RCC and SBRT. It was implemented online via Google Forms and sent to clinicians identified as key opinion leaders.
The Delphi consensus methodology was applied and involved sending three rounds of questionnaires to the experts. After the conclusion of each round, a fully anonymised summary of their and others’ responses was provided to the participants, in order to drive the panellists towards the consensus. The agreement threshold was set at 80%.
Results
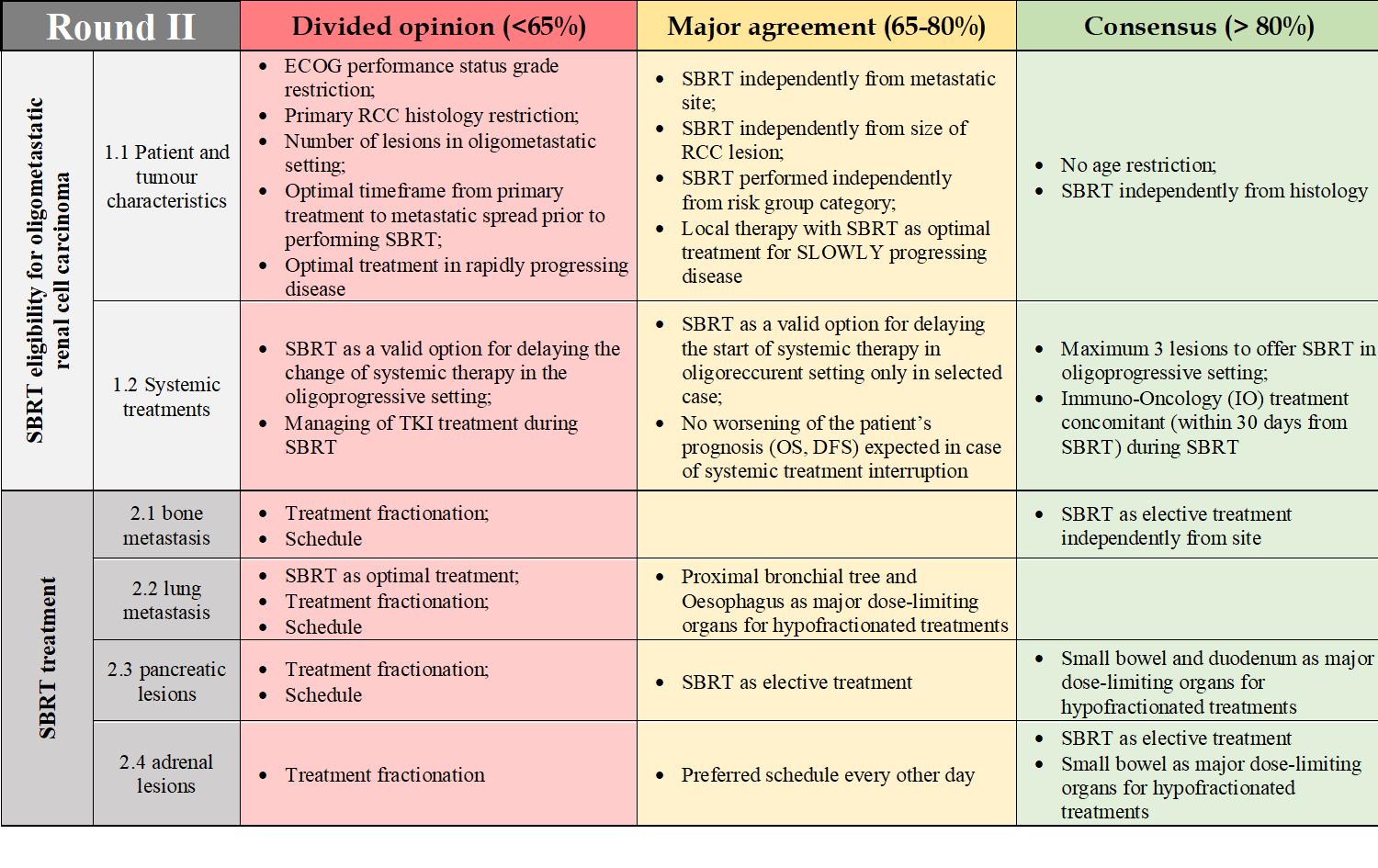
Among the 43 contacted professionals, a total of 25 experts (58%) agreed to participate. Responders, characteristics are reported in Table 1. Two out of the three planned rounds of responses have been completed. At the end of the first round, participants were able to find consensus on 7 out of 52 clinical questions. Specifically, SBRT was indicated as the elective treatment choice for RCC bone metastases (80%) and for adrenal lesions (88%) independently from site. Small bowel, duodenum, and stomach were the major dose limiting organs for both pancreatic and adrenal lesions, with the addition of kidney for the latter. At the end of the second round, one further question reached the consensus, with 83% of responders agreeing on an upper threshold of 3 lesions for SBRT in the oligoprogressive setting. A summary of the main areas of agreement and disagreement is reported in Table 2.
Table 1. General information about the writing committee population
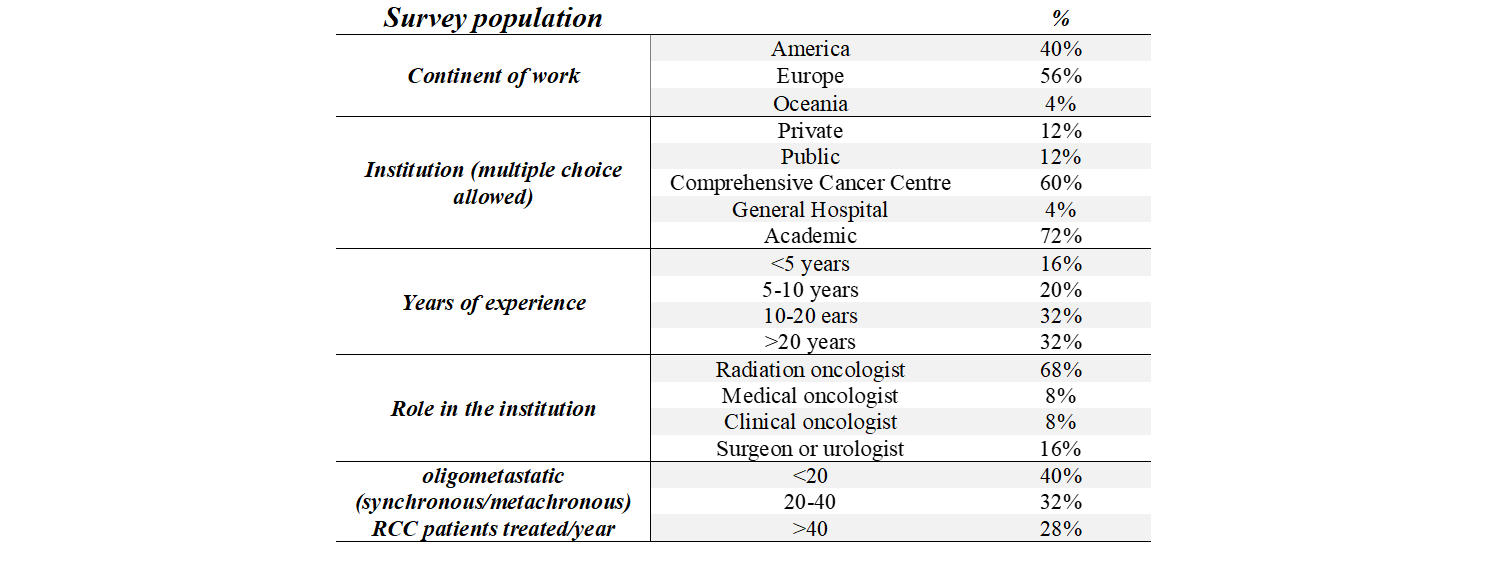
Table 2: Areas of divided opinion (agreement < 65%), major agreement (65% < agreement < 80%), and consensus (agreement > 80%) in section 1 and 2 of the questionnaire at the end of round II.
Conclusion
The present ESTRO ACROP study represents one of the first efforts to achieve consensus on some critical aspects in SBRT in oligometastatic RCC, which is currently a controversial topic. This ESTRO ACROP consensus may serve as practical guidance for the use of SBRT in oligometastatic RCC, and indicate the main areas where disagreement still persists, possibly guiding future efforts in filling knowledge gaps.