Patterns of Recurrence & Outcomes in Bladder Cancer Treated with Radiotherapy & Radiosensitisation
Taha Lodhi,
United Kingdom
OC-0432
Abstract
Patterns of Recurrence & Outcomes in Bladder Cancer Treated with Radiotherapy & Radiosensitisation
Authors: Taha Lodhi1, Yee Pei Song1, Thiraviyam Elumalai2, Andrew Hudson1, John Logue1, James Wylie1, William Croxford1, Maria Serra1, Anna Tran1, Ruth Conroy1, Peter Hoskin3, Ananya Choudhury4
1The Christie NHS Foundation Trust, Manchester Academic Health Science Centre, Department of Clinical Oncology, Manchester, United Kingdom; 2Cambridge University Hospitals NHS Foundation Trust, Department of Clinical Oncology, Cambridge, United Kingdom; 3Cancer Centre, Mount Vernon Hospital; Manchester Cancer Research Centre, University of Manchester, Department of Clinical Oncology; Division of Cancer Science, London/ Manchester, United Kingdom; 4The Christie NHS Foundation Trust, Manchester Academic Health Science Centre; Manchester Cancer Research Centre, University of Manchester, Department of Clinical Oncology; Division of Cancer Science, Manchester, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
Radiotherapy plays a central role in muscle-invasive bladder cancer (MIBC) when combined with radiosensitisation and is an established standard of care. Given the different modes of action of radiosensitisers, we hypothesised that patterns of recurrence might differ between GemX (weekly low-dose gemcitabine) and carbogen and nicotinamide (BCON).
Material and Methods
With institutional approval, clinical, pathological, and treatment parameters were obtained for a retrospective cohort study of patients treated with BCON or GemX radiotherapy between 2017-2021 based on clinician preference. Clinician-reported toxicity was assessed using RTOG grading during radiotherapy, 6 weeks, and 12 months after. Cross-sectional imaging and cystoscopy determined recurrence, progression-free survival (PFS), and cancer-specific survival (CSS). Subgroup differences were compared using T and χ2 tests. Survival was analysed by Kaplan-Meier, log-rank, and multivariate Cox proportional hazard regression. Statistical analysis was conducted using R (R Core Team (2022)).
Results
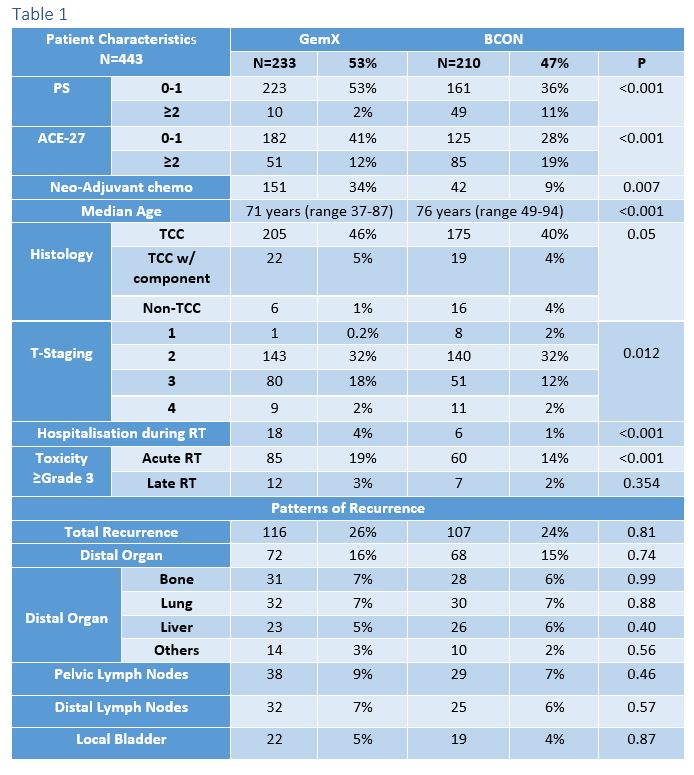
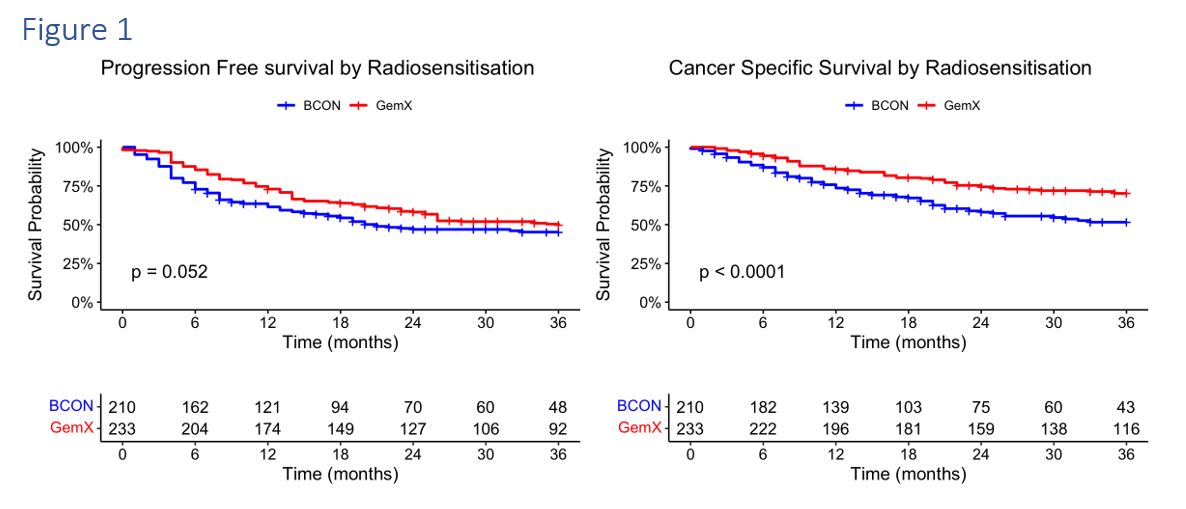
Of the 443 patients analysed, 210 were treated with BCON; 233 were treated with GemX. The median follow-up was 32m (6-66). Patient characteristics are shown in Table 1. BCON patients were older, significantly less fit, received less neoadjuvant chemotherapy, and had more non-TCC cancers and less T3 staging. Treatment completion rates were 96% for GemX and 95% for BCON. There were significantly more hospitalisations (4%) and acute grade 3 toxicities (19%) after GemX radiotherapy (p<0.001) but there was no difference in late toxicity (p=0.354). 3-month cystoscopy post radiotherapy demonstrated complete response in 92% (151/164) of BCON and 90% (187/208) of GemX. Overall, 107 (24%) progressed after BCON and 116 (26%) following GemX. Patterns of recurrences are demonstrated in Table 1 and did not differ significantly between BCON and GemX. 3-year PFS was 45% for BCON and 49% for GemX (HR 0.77, 95%CI (0.59-1.0, p=0.052); 3-year BCON CSS was 52% and 70% for GemX (HR 0.53, 95%CI 0.38-0.73, p<0.001) as shown in Figure 1. When adjusted for clinical factors (neoadjuvant chemotherapy, gender, age, performance status, T-stage, ACE-27, and carcinoma in-situ) in a multivariate Cox model, both PFS (HR 0.70 95%CI 0.50-0.96, p=0.027) and CSS (HR 0.56, 95%CI 0.38-0.82, p=0.0026) were significant in favour of GemX.
Conclusion
Our real-world data is comparable to published BCON and GemX outcomes and both methods of radiosensitisation have acceptable toxicity and high treatment completion rates. Patterns of recurrence were similar regardless of radiosensitisation. CSS was superior in GemX patients, but differences in outcomes for GemX and BCON are likely explained by differing use of neoadjuvant chemotherapy and patient factors. Given that this is non-randomised data, direct comparisons of both treatments should be treated with caution.