A deterministic algorithm for patient-specific quality assurance in online adaptive proton therapy
Tiberiu Burlacu,
The Netherlands
OC-0117
Abstract
A deterministic algorithm for patient-specific quality assurance in online adaptive proton therapy
Authors: Tiberiu Burlacu1, Danny Lathouwers1, Zoltán Perkó1
1HollandPTC consortium – Erasmus Medical Center (ErasmusMC), Rotterdam, Holland Proton Therapy Center (HollandPTC), Delft, Leiden University Medical Center (LUMC), Leiden, and Delft University of Technology (TU Delft), Delft, The Netherlands, Delft University of Technology, Department of Radiation Science and Technology, Delft, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
To develop a physics-based deterministic proton transport algorithm (DPQA) that can cheaply but accurately compute dosimetric changes based on CT and log-files derived perturbations, serving thus as an independent patient-specific quality assurance tool.
Material and Methods
Online adaptive workflows necessitate accurate and fast solutions of the Linear Boltzmann Equation (LBE). Through the Continuous Slowing Down Approximation with energy straggling, the Fermi-Eyges approximation and assuming energy separability, the LBE was simplified into two partial differential equations (PDEs): the one-dimensional Fokker-Planck (FPE) and the Fermi-Eyges equations (FEE). The FPE was solved numerically using the discontinuous Galerkin Finite Element Method in energy and the Crank-Nicholson method in depth, correctly modelling heterogeneities. The FEE has an analytical Gaussian solution whose coefficients are determined by depth-wise integrating the transport cross section. To handle lateral heterogeneities a beam-splitting scheme with concentric rings surrounding the original beam was implemented. The beamlet weights, spreads and distances from the central axis were optimized and their number increased with the distance from the central axis. The PDE based methodology allows applying adjoint theory, yielding fast determination of the energy deposited (or other metrics of clinical interest) in a variable region of interest (ROI) due to perturbations (anatomy, beam delivery) without repeated re-calculations.
Results
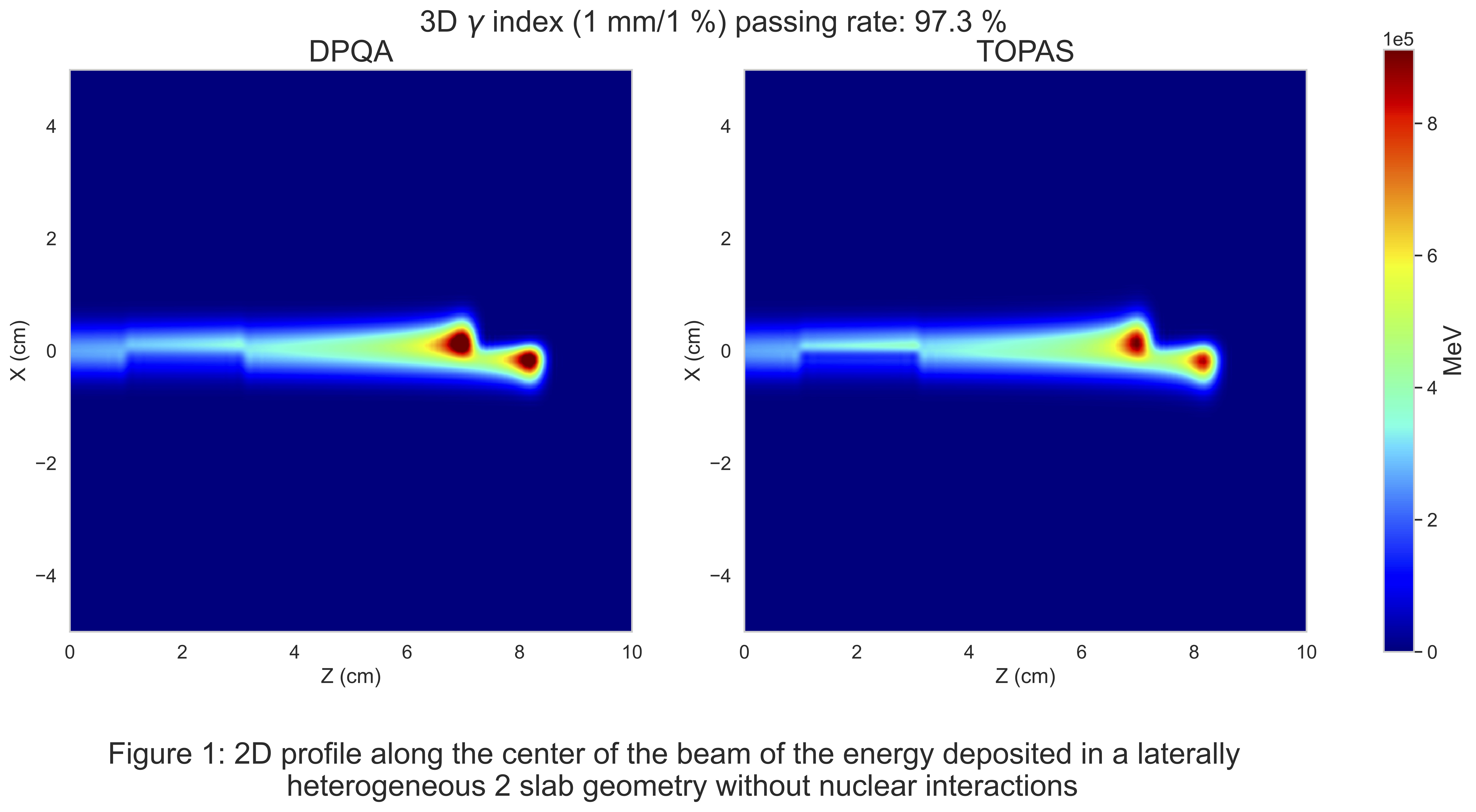
Sub-second beamlet run times were achieved. An integrated depth dose (IDD) comparison in a homogeneous water tank versus TOPAS Monte Carlo calculations (considered the gold standard) showed good overlap in the Bragg peak and an enhanced IDD in the entrance region compared to Bortfeld’s algorithm due to the modelling of nuclear interactions. Disabling nuclear interactions results in a 3D gamma index (1 mm/1 %) passing rate of 100%. A laterally heterogeneous phantom was also tested, in which two slabs (of ± 400 HU) are placed between 1 and 3 cm in depth in a tank with a base composition of 10 HU. A 3D gamma index (1 mm/1%) resulted in 97.3 % passing rate (Figure 1).
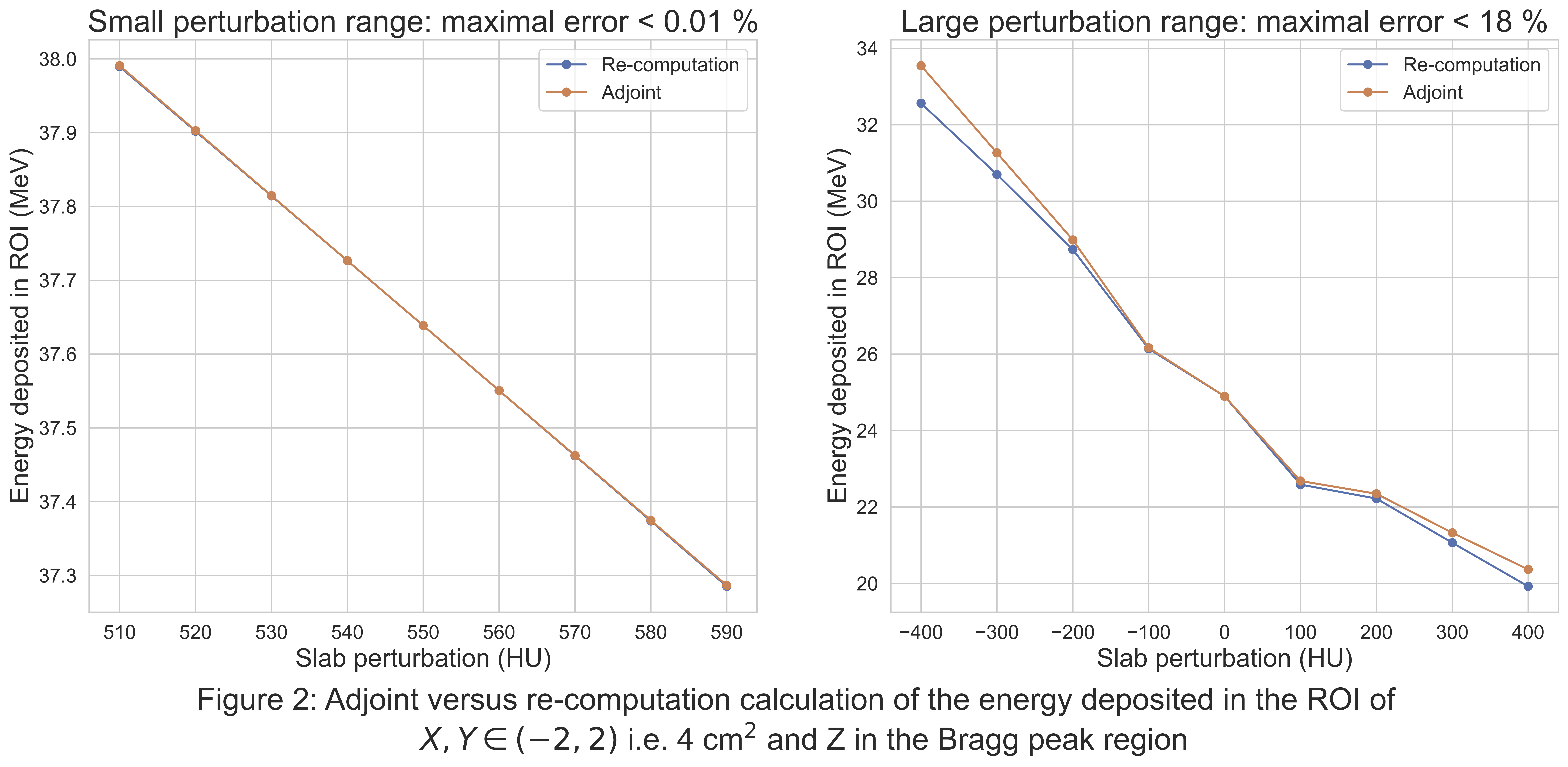
As perturbations for the adjoint component, slabs of different thickness and positions were placed along the depth of the tank and the energy deposited in the ROI was computed. Near overlap was achieved for medium perturbation ranges (±40 HU) and large HU ranges (±1000 HU) yielded <18% maximal errors (Figure 2).
Conclusion
The adjoint-physics based model provided TOPAS-like accuracy for electromagnetic interactions in sub-second beamlet runtimes with acceptable errors over large HU ranges without requiring expensive re-calculations and typical limitations (distributions shifts, out-of-distribution samples) of deep learning models.