Assessing the clinical impact of brachytherapy source tracking uncertainties of a novel IVD system
Teun van Wagenberg,
The Netherlands
OC-0635
Abstract
Assessing the clinical impact of brachytherapy source tracking uncertainties of a novel IVD system
Authors: Teun van Wagenberg1, Robert Voncken1, Celine Van Beveren1, Frank Verhaegen1, Gabriel Paiva Fonseca1
1Maastricht University Medical Centre+, Department of Radiation Oncology (Maastro), Maastricht, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Even though the outcomes reported for high-dose-rate (HDR) brachytherapy (BT) are very good, there is currently no widespread method for treatment verification. Therefore, we have been working on an in vivo dosimetry (IVD) system using an imaging panel (IP) for source tracking. However, the translation of measured dwell positions and times to more clinically relevant parameters like DVHs is missing. This study describes how we can use software to calculate the dose from our source tracking approach and use this to determine the clinical relevance of the system uncertainty and the dosimetric impact of treatment errors.
Material and Methods
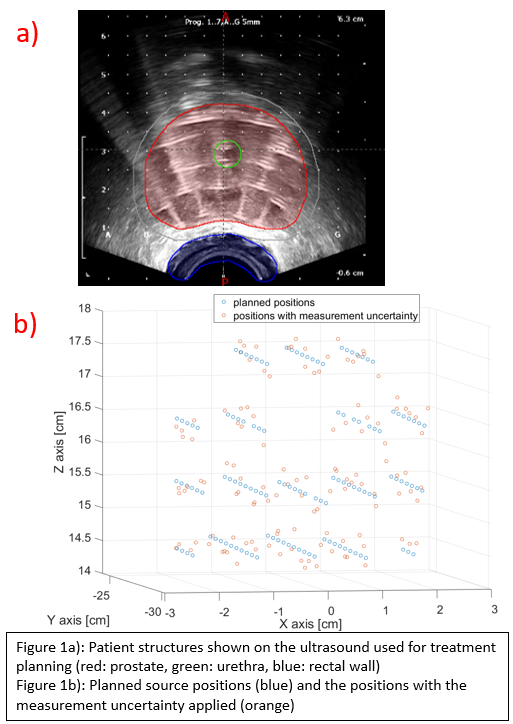
The target volume, OAR (urethra and rectum) and catheters with dwell positions were exported from a real prostate case (figure 1a). Measured data from earlier studies in 3D printed phantoms is used to create a distribution of the random errors that are caused by the uncertainty of our IVD system, which has been determined to be around 1.7±0.7mm in 3D. These random measurement errors are applied to the dwell positions from the patient plan (figure 1b). Another distribution of 3.4±1.2mm was used to simulate a situation where the measurement would be worse. A dose calculation tool based on the TG-43 formalism was created to determine the dose from dwell positions and dwell times, as most commercial TPS systems don’t allow for straightforward dose calculation from experimental data. Using the structures from the patient case, DVHs can then be determined. Similarly, treatment errors can be simulated to test if their clinical relevance could be assessed. Treatment errors can be introduced as systematic errors, for example when the entire implant shifts in a certain direction all dwell positions are shifted with the same value.
Results
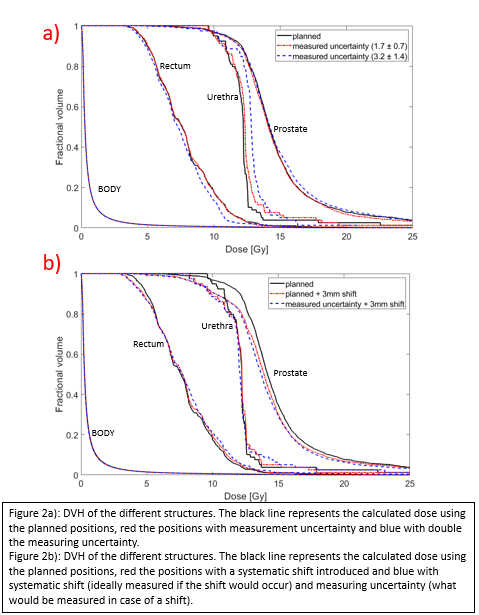
The DVH of the positions with measurement uncertainty show a small difference with the DVH of the planned positions in the prostate and rectum (figure 2a). However, it was more pronounced in structures that are close to high gradient regions such as the urethra. Increasing the magnitude of the errors still has acceptable DVHs for the rectum and target volume, but the dose in the urethra becomes much higher. Figure 2b shows that even with the measurement uncertainties, the DVH that our system would measure is much closer to the DVH that would be expected from this shift than to the planned positions, illustrating that the clinical impact of such shifts can be measured with our system.
Conclusion
Using the software the source tracking measurements can be translated into clinical parameters, and the clinical relevance of deviations can be assessed. Results show that the uncertainty of our IVD system has a small impact on the DVHs, even when an offset happened during the treatment. However, more clinical cases with different treatment errors still have to be evaluated. This is essential to fully understand the uncertainties of the proposed IVD method in a clinical context, and help define action limits for error detection.