Quantitative imaging of hypoxic and immunologically cold CAIX+ areas in syngeneic mouse models
Daan Boreel,
The Netherlands
OC-0093
Abstract
Quantitative imaging of hypoxic and immunologically cold CAIX+ areas in syngeneic mouse models
Authors: Daan Boreel1, Paul Span1, Hans Peters1, Annemarie Kip2, Milou Boswinkel2, Gosse Adema1, Sandra Heskamp3, Jan Bussink1
1Radboudumc, Radiation Oncology, Nijmegen, The Netherlands; 2Radboudumc, Medical Imaging, Nijmegen, The Netherlands; 3Raboudumc, Medical Imaging, Nijmegen, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Limited diffusion of oxygen in combination with increased oxygen consumption and a chaotic vasculature leads to chronic hypoxia in most solid malignancies. This scarcity of oxygen is known to induce radioresistance, but also leads to a more immunosuppressive immune phenotype. CAIX is an enzyme functioning as a catalyzer for acid export in hypoxic cells upregulated by hypoxia master regulator HIF-1α, and is an endogenous biomarker for hypoxia. We developed a radiolabeled antibody that recognizes murine CAIX ([111In]In-DTPA-mCAIX) to visualize and monitor chronic hypoxia in syngeneic mouse models.
Material and Methods
Three syngeneic tumor models were used in this study (B16ova, B16F1, MOC1). Cells were cultured at hypoxic conditions of 1% O2 and CAIX expression was determined by FACS analysis or cells were incubated with 700 Bq 111In-anti-mCAIX for 2 hours at 37°C after which cell-associated activity was measured by radioactivity counting. In vivo distribution of tracer uptake, CAIX and immune cells were visualized using ex vivo autoradiography and immunohistochemistry. Image analysis was performed by parametric mapping in ImageJ. In vivo SPECT/CT scans were acquired using the mouse HS 1.0 mm pinhole collimator (U-SPECTII/CT) or the GP-M 0.60 mm pinhole collimator (U-SPECT6/CT), followed by a CT scan. Scans were analyzed using VivoQuant.
Results
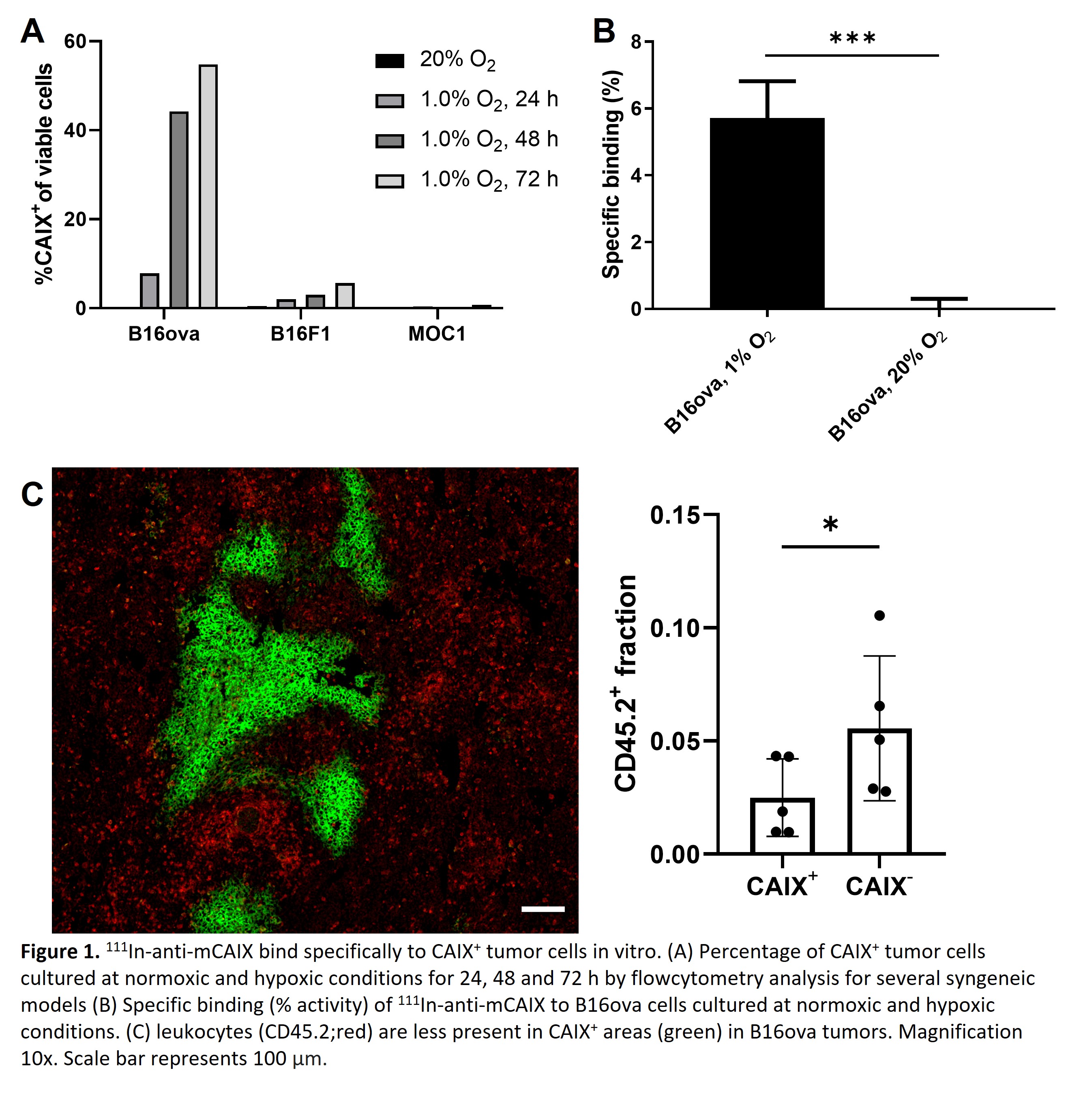
In vitro, inducibility of CAIX expression was determined for several syngeneic tumor models (B16ova; high inducible, B16F1; intermediate inducible, MOC1; low inducible) (Fig 1A). [In111]-DTPA-mCAIX showed specific binding to B16ova cells when cultured at 1% O2 (5.7±0.9%), but not to cells cultured at 20% O2 (0.02±0.3%), suggesting it is suitable for in vivo imaging of CAIX+ hypoxic areas (Fig 1B). These areas are clinically relevant because they are poorly infiltrated by CD45.2+ immune cells in B16ova tumors (fraction: 0.025±0.015) compared to CAIX- areas (fraction: 0.054±0.032) (Fig 1C). In vivo, CAIX expression could be visualized by SPECT using [In111]-DTPA-mCAIX (Fig 2A). Autoradiography and immunohistochemistry of tumor sections showed a strong spatial correlation of CAIX expression with [In111]-DTPA-mCAIX localization for CAIX+ tumor models (B16ova; r=0.55±0.21, B16F1; r=0.46±0.12) but not for CAIX- tumors (MOC1; r=-0.20±0.17) (Fig2B). Quantification of CAIX+ fraction on SPECT/CT images was significantly higher in B16ova (0.18±0.03) compared to B16F1 (0.07±0.05) and MOC1 (0.03±0.04) tumors.
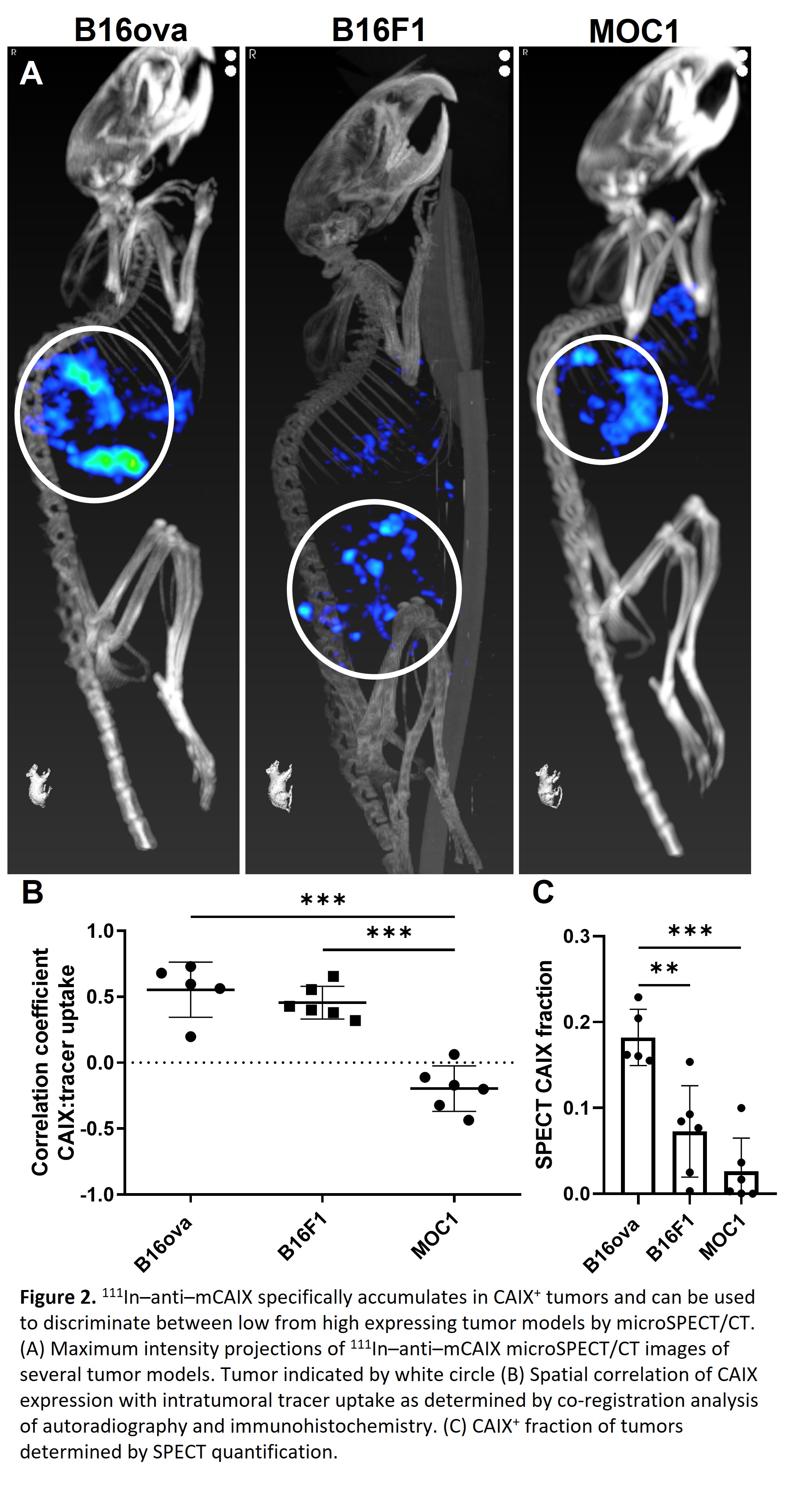
Conclusion
The hypoxia related marker CAIX can be used to visualize hypoxic and immunological cold areas in syngeneic mouse models using the SPECT-radiotracer [In111]-DTPA-mCAIX. We show this technique is able to distinguish CAIX high from CAIX low tumors by ex vivo analysis and in vivo SPECT/CT imaging. In the future, this technique could be used to distinguish hypoxic from non-hypoxic tumors before or during hypoxia targeted or reducing treatment and thereby help optimizing this strategy to improve immuno- and radiotherapy efficacy in preclinical models.