External validation of a PET radiomic model predicting outcome after RT for oropharyngeal cancer
PD-0400
Abstract
External validation of a PET radiomic model predicting outcome after RT for oropharyngeal cancer
Authors: Martina Mori1, Chiara Deantoni2, Gabriele Palazzo1, Michela Olivieri1, Emiliano Spezi3, Anna Chiara2, Maria Picchio4, Antonella Del Vecchio1, Nadia Gisella Di Muzio2, Claudio Antonio Fiorino1, Italo Dell'Oca2
1IRCCS San Raffaele Scientific Institute, Medical Physics, Milan, Italy; 2IRCCS San Raffaele Scientific Institute, Radiotherapy, Milan, Italy; 3School of Engineering Cardiff University, Medical Physics, Cardiff, United Kingdom; 4IRCCS San Raffaele Scientific Institute, Nuclear Medicine, Milan, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
To externally validate the 18Fluorodeoxyglucose Positron Emission Tomography (18F-FDG-PET ) radiomic-based model suggested by Martens et al. (EJNMI Research (2020) 10:102) predicting overall survival (OS) in patients with oropharyngeal cancer treated with chemo-radiotherapy.
Material and Methods
Complete outcome data and pre-radiotherapy PET images of 67 patients treated at our Institute between 2011 and 2021 according to an internal protocol delivering moderate hypo-fractionation (66 Gy in 30 fr) were available. Image processing (voxelization and binning) was performed according to the procedures described by Martens et al. and tumors were segmented using a previously validated semi-automatic method with the software MIM (v.6.9.6). Four-hundred and fifty radiomic features (RF) were extracted according to IBSI (Image Biomarker Standardization Initiative) guidelines, using the SPAARC software. Martens et al. condensed the predictive RF in 8 independent meta-factors (F), consisting of a combination of selected RF with variable importance weight. In particular, the combination of two F, named F1 and F5, with SUV_max, SUV_mean and HPV status resulted in the predictive model of OS. The risk of death was computed by the formula suggested by Martens et al and used to stratify risk according to the best cut-off derived from ROC analysis. In addition to the Martens’ study, PET based tumor Volume was tested separately in Univariate analysis in order to quantify its own contribution respect to the model performance. Concordance index (CI) for the Martens model and using the volume was calculated and compared.
Results
With a median follow-up of 35 months, 9 deaths were registered. The risk computed as combination of F1, F5, SUV_max, SUV_mean and HPV status was confirmed to be associated to OS (p=0.021, CI=0.60 (0.47-0.72)). The Youden criterion of the ROC analysis was used showing high ability in stratifing patients in risk classes as depicted by the Kaplan Meier curve reported in Figure 1 (p=0.0132, HR=7.64, 95%CI=1.53-38.14). The 3-year OS were 92% (95%CI: 85-100%) vs 67% (30-86%) for low and high-risk groups respectively. Also the Volume was found associated to OS (p=0.007, CI=0.61 (0.48-0.73)) with high ability in patients' stratification, as depicted by the Kaplan Meier curve reported in Figure 2 (p=0.0307, HR=4.31, 95%CI=1.14-16.19). The 3-year OS were 95% (95%CI: 85-100%) vs 75% (60-85%) for low and high-risk groups respectively.
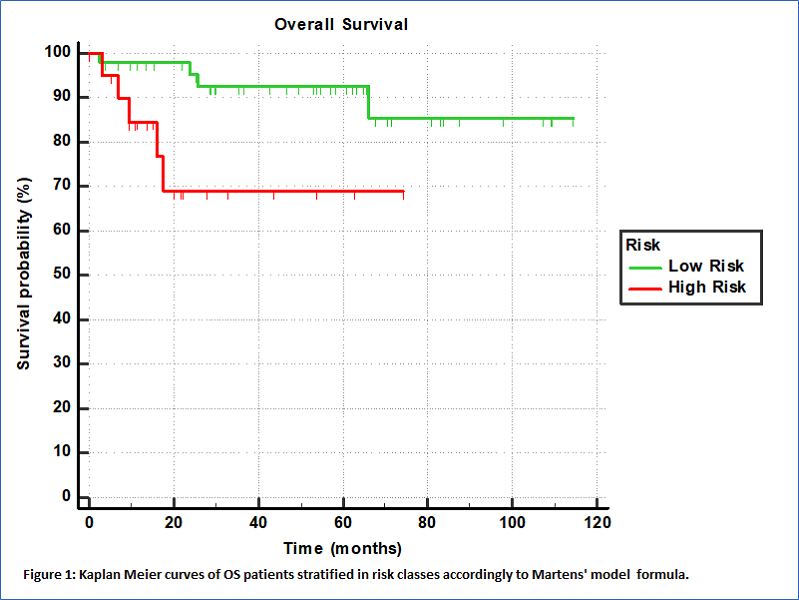
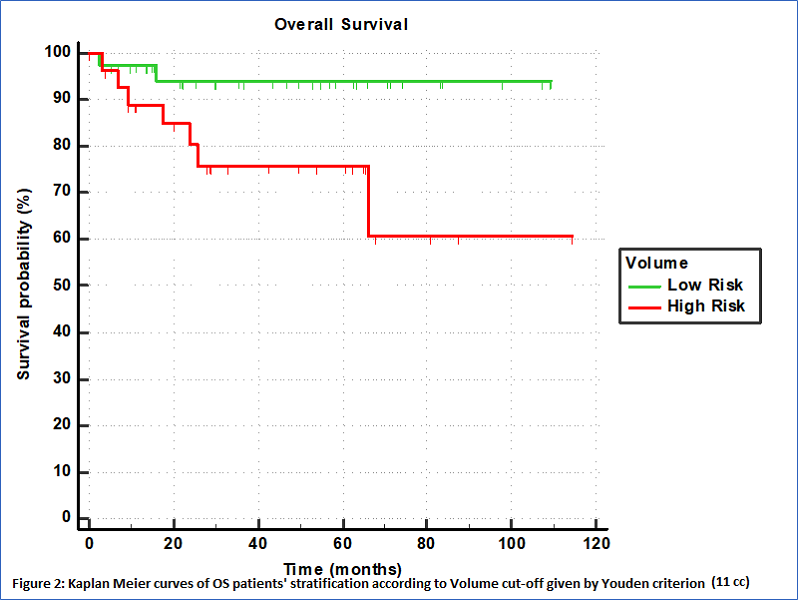
Conclusion
A previously published model was externally confirmed in predicting OS, despite different Institute/scanners, delivered dose and patient’s characteristics. Similar predictive performances were obtained if using only the PET-based tumor volume, suggesting that the additional benefit of more complex PET RF-based signatures remains to be demonstrated. Multiple validations like the current one may help in corroborating the generalizability of radiomic-based models, allowing for personalized risk stratification and optimization of personalized cancer care.