Radiosensitization by DNA Damage response inhibitors correlates with Replication stress & HR scores
PD-0826
Abstract
Radiosensitization by DNA Damage response inhibitors correlates with Replication stress & HR scores
Authors: Waisse WAISSI1, Selma Rebus2, Ross Carruthers3, Mark Jackson4, Lynn McGarry5, Stephen Dreyer2, David Chang2, Anthony Chalmers2
1School of Cancer Sciences, Wolfson Wohl Cancer Research Centre, University of Glasgow, Glasgow, United Kingdom; 2School of Cancer Sciences, Wolfson Wohl Cancer Research Centre, University of Glasgow, Glasgow, United Kingdom; 3School of Cancer Sciences Wolfson Wohl Cancer Research Centre, University of Glasgow, Wohl Cancer Research Centre, University of Glasgow, Glasgow, United Kingdom; 4School of Cancer Sciences Wolfson, Wohl Cancer Research Centre, University of Glasgow, Glasgow, United Kingdom; 5Cancer Research UK, Beatson Institute, Glasgow, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
Pancreatic ductal adenocarcinoma (PDAC) is a devastating disease with 5-year overall survival of 9% for all stages. A key determinant of cellular radiosensitivity is the capacity to repair lethal DNA double-strand breaks (DSBs) that are induced by ionizing radiation. Targeting proteins involved in the DNA damage response (DDR) is therefore a promising therapeutic strategy, but predictive biomarkers are required to maximise clinical benefit. We conducted an in vitro study to determine the extent to which clinically relevant inhibitors of key DDR proteins (ATR, PARP and ATM) radiosensitized a panel of PDAC cell lines. We then looked for molecular alterations that associated with the magnitude of radiosensitization across the panel.
Material and Methods
Eight patient derived PDAC cell lines were treated with small molecule inhibitors of ATR (AZD6738 : 20nM - 1µM), PARP (olaparib : 20nM - 1µM) or ATM (AZD1390: 1nM - 100nM) alone and in combination with radiation (0 to 6 Gy). Responses were evaluated by proliferation (8 cell lines) and clonogenic assays (7 cell lines). Sensitizer enhancement ratios (SERs) were calculated from mean inactivation doses, which were determined by integration of the LQ equation. Previously obtained genomic and transcriptomic data were then interrogated to identify possible biomarkers of radiosensitization. Transcriptomic replication stress (RS) scores were generated by totaling the score of GO Terms involved in DDR and cell cycle control (sig.score function from R package genefu). Three DDR deficiency scores derived from whole genome sequencing data were also assessed (BRCA signature, Structural Variant, HRDetect).
Results
The ATM inhibitor AZD1390 had no single agent activity but was a potent radiosensitizer at low concentrations and in all cell lines (5nM, SER 1.30-1.83; Fig.1). SER value for AZD1390 correlated with RS score as a continuous variable (p=0.01; Fig. 2). In contrast, the radiosensitizing effects of olaparib and AZD6738 were dependent on drug concentration and cell line. Cell lines harbouring homologous recombination deficiency (BRCA signature) and high RS score were radiosenstized by olaparib (SER :1.75 vs 1.09, p=0.01). and ATRi (SER : 1.35 vs 1.05) respectively (Fig. 2).
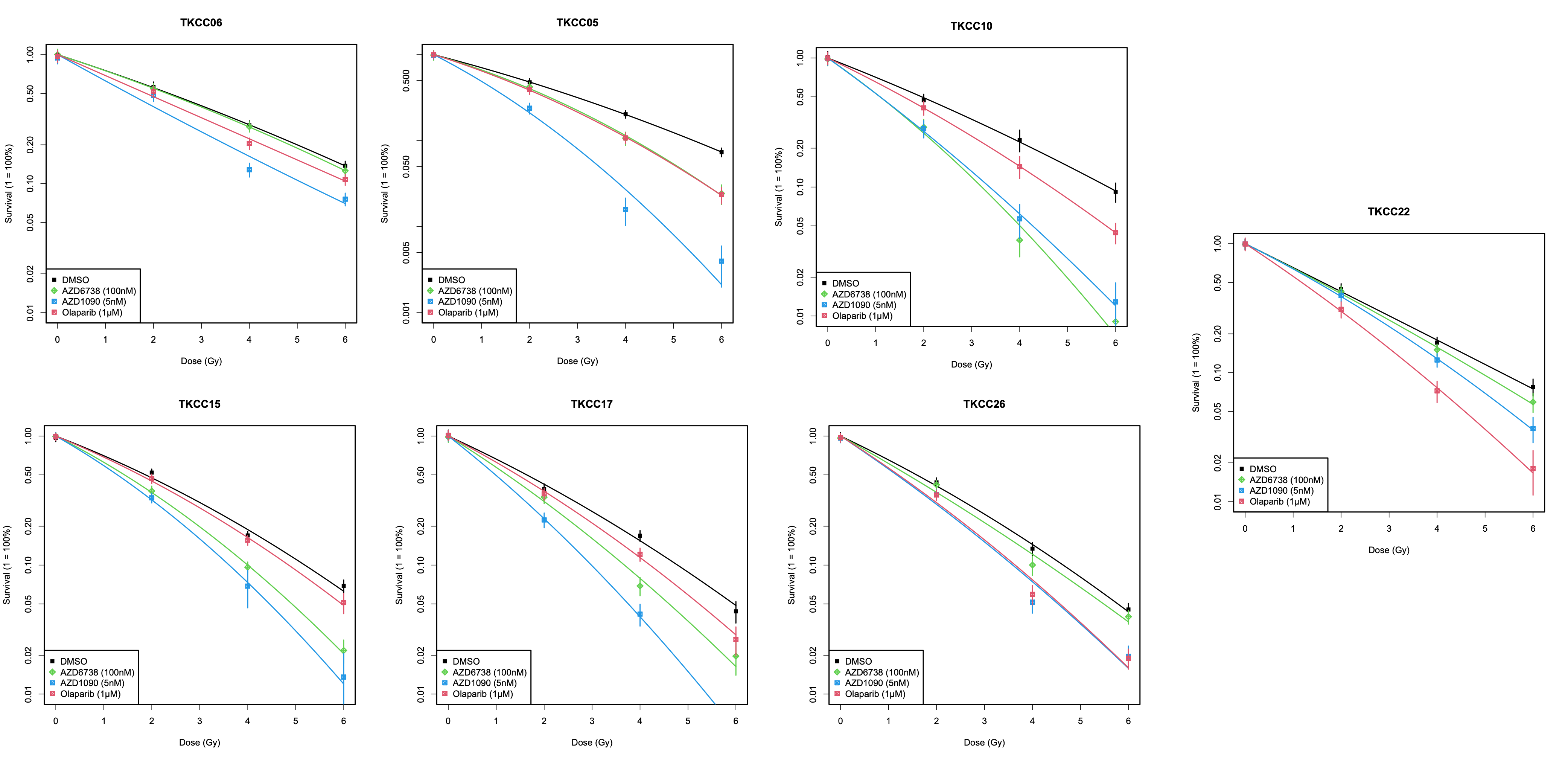
Figure 1 : Survival curves showing radiosensitization of 7 patient derived PDAC cell lines with ATRi(green), ATMi(Blue), PARPi(Red)
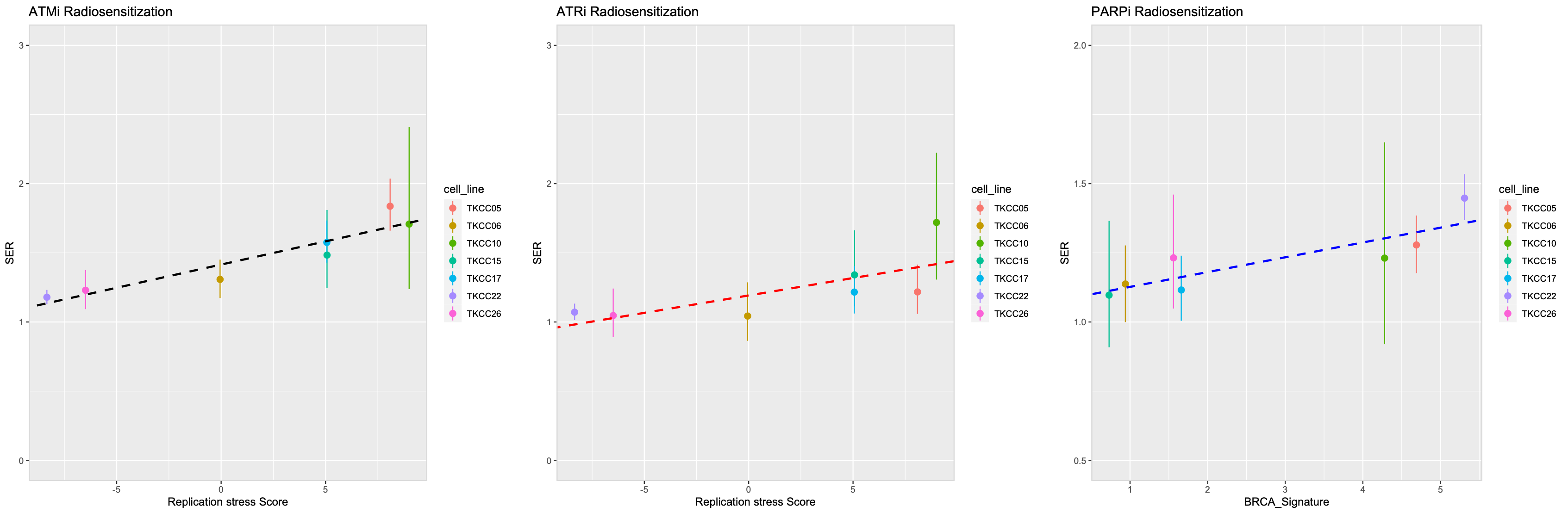
Figure 2 : Correlation between Replication stress Score and Homologous recombination repair deficiency with ATRi/ATMi and PARPi radiosensitization
Conclusion
DDR inhibitors radiosensitized PDAC with the magnitude of radiosensitization depending on Replication Stress and DDR deficiency. These data provide potential therapeutic strategies in the radioresistance setting.