Spatial distribution of CD8-positive T cells predicts radiation response in oropharyngeal cancer
PD-0816
Abstract
Spatial distribution of CD8-positive T cells predicts radiation response in oropharyngeal cancer
Authors: Justus Kaufmann1, Maximilian Haist2,3,4, Ivan Kur5, Stefanie Zimmer6, Stephan Grabbe2, Heinz Schmidberger1, Andreas Weigert5, Arnulf Mayer1
1University Medical Center of the Johannes-Gutenberg-University, Department of Radiation Oncology and Radiotherapy, Mainz, Germany; 2University Medical Center of the Johannes-Gutenberg-University, Department of Dermatology, Mainz, Germany; 3Stanford University School of Medicine, Department of Pathology, Stanford, USA; 4Stanford University School of Medicine, Department of Microbiology & Immunology, Stanford, USA; 5Faculty of Medicine Goethe-University Frankfurt, Institute of Biochemistry I, Frankfurt, Germany; 6University Medical Center of the Johannes-Gutenberg-University, Institute of Pathology, Mainz, Germany
Show Affiliations
Hide Affiliations
Purpose or Objective
Effective anti-tumor immune responses are mediated by CD8-positive cytotoxic T cells (CTL) and require organized, spatially coordinated interactions within the tumor microenvironment (TME). Understanding coordinated T-cell-behavior and deciphering mechanisms of radiotherapy resistance mediated by tumor stem cells will advance risk stratification of oropharyngeal cancer (OPSCC) patients treated with primary chemoradiotherapy (RCTx).
Material and Methods
To determine the role of CTL and tumor stem cells for response to RCTx, we employed 7-plex immunofluorescence stains on a dedicated tissue microarray of pre-treatment biopsies from 86 advanced OPSCC patients. The TMA was constructed from 1.2mm diameter cores punched from two representative regions of the invasive tumor front of the tissue blocks. Seven-color multiplex fluorescence stains for the antigens pan-Cytokeratin, p16INK4a, CD271, PD-L1, Ki67 and CD8 were performed using the Opal Polaris 7-Color Manual Detection Kit. Single-cell-based analyses of multiplex stains were carried out for all TMA cores in the open-source image analysis software QuPath (Figure 1). Distribution of cell phenotypes within the TME was explored using the R-package Spatstat. Resulting quantitative single-cell data were finally correlated with clinical parameters, such as patient demographics, smoking and alcohol consumption, treatment regimens and survival data. Primary endpoints included best response to RCTx and overall survival (OS).
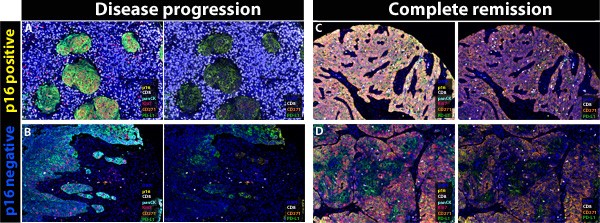
Figure 1: Representative illustrations showing the role of spatial distribution of CD8 T cells within the tumor microenvironment of 4 advanced OPSCC patients.
Results
Our observations demonstrate that a strong CTL-infiltration into the epithelial neoplastic tumor compartment (HR for overall survival, OS: 0.35; p<0.001) and the expression of PD-L1 on CTL (HR: 0.36; p<0.001) were both associated with a significantly better response and survival upon RCTx that was confirmed in a multivariate Cox-regression model. By contrast, overall CTL infiltration, regardless of the affected compartment, was not associated with response to therapy or survival (Figure 2). As expected, p16 expression was a strong predictor of improved OS (HR: 0.38; p=0.002) and correlated with overall CTL infiltration (r: 0.358, p<0.001). Tumor cell proliferative activity and the expression of the tumor stem cell marker CD271 were not associated with response or survival.
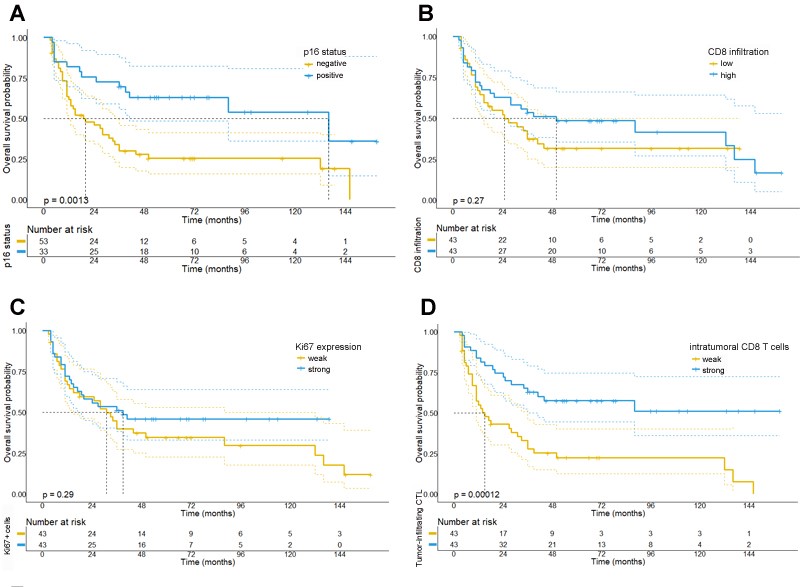
Figure 2: Kaplan Meier survival plots depicting the prognostic significance of the investigated biomarkers for OPSCC patients.
Conclusion
In this study we demonstrate the clinical relevance of the spatial organization and the phenotype of CTL within the TME. In particular, we found that CTL infiltration specifically into the epithelial neoplastic cell compartment was an independent predictive marker for the response to RCTx. Conversely, tumor cell proliferation and the expression of the stem cell marker CD271 showed no independent predictive effect for response to RCTx and require further study.