MR-guided daily adapted SBRT for prostate cancer: Acute and late patient reported outcomes
Killian Nugent,
United Kingdom
PD-0573
Abstract
MR-guided daily adapted SBRT for prostate cancer: Acute and late patient reported outcomes
Authors: Killian Nugent1,2, Ami Sabharwal3, Carla Perna3, Prantik Das4, Nicola Dallas5, Philip Camilleri5
1Genesiscare, Radiation Oncology, Oxford, United Kingdom; 2Genesiscare, Radiation Oncology, Oxford, Ireland; 3Genesiscare, MRI Linac, Oxford, United Kingdom; 4Genesis Care, MRI Linac, Oxford, United Kingdom; 5GenesisCare, MRI Linac, Oxford, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
To record patient reported GI & GU adverse events (PRO CTC AE), in the acute and late effects setting, amongst prostate cancer patients who underwent MRI-guided adaptive SABR.
Material and Methods
Patients who completed a baseline 12 item PRO-CTC AE (AE), before undergoing 36.25 Gy in 5 fractions by MRI-guided adaptive SABR, were subsequently followed up with the same graded questionnaire: none; mild/rarely; moderate/occasionally; severe/frequently; very severe/almost constantly at set time points. Latest PSA levels was recorded. The % of patients who reported no change from their baseline AE or reported a new ≥ ‘frequent’ or’ severe’ AE grade during follow up was calculated. For individual patients, the maximum AE grade for each PRO-CTC AE item was recorded. The % of toxicity levels for each separate AE item at set time points were calculated.
Results
Median follow-up was 27 months (range: 6-34 months) for 69 patients (median age 73). The median pre-RT PSA was 7.5 mmol/l (4.5-32 mmol/l), median T Stage T2b (T0-T3b) and the median Gleason Score of 7 (3+4, range: 6-9). 29% of patients were in the high-risk category. No patient had biochemical failure (median PSA 0.42) at time of follow up.
In the CTC PRO AE GI categories, most men reported no change from baseline toxicity level in the follow up period; diarrhoea 84%, constipation 84%, faecal incontinence 81%, pain on opening bowels 72%, general pain 83%, GI bleeding 88%. There was no new “frequent” GI bleeding. 45% of patients reported at worst some mild or moderate diarrhoea (Fig 1) with new ≥ “severe” diarrhoea reported in 9% of patients (Tab.1) peaking at 24 months but absent at > 33 months (Fig. 2.1).
Similarly, in the urinary AE categories, apart from urinary urgency, >80% of men reported no change from baseline toxicity during follow up. 20% of men reported ≥ “frequent” urinary urgency with incidence peaking during the first month follow up but absent in patient reporting at >33 months (Fig 2.2). No man reported new “frequent” blood in urine at any point. 7% of men reported ≥ “quite a bit” of urine leakage during follow up peaking as a late effect during the 13–19-month period (Fig 2.4). New “severe” sexual dysfunction was seen in 25% of patients and was persistent at > 33 months with 68% of patients having received ADT.
PRO-CTC AE (AE)
| No increase in AE Grade from Baseline
| New ≥ “Frequent or Severe” AE reported
|
| Diarrhoea | n=58 (84%) | n=6 (9%) |
| Constipation | n=58 (84%) | n=2 (3%) |
| Faecal incontinence | n=56 (81%) | n=1 (1%) |
| Pain on opening bowel | n=50 (72%) | n=4 (6%) |
| General pain | n= 57 (83%) | n=4 (6%) |
| GI bleeding | n=61 (88%) | n=0 (0%) |
| Painful urination | n=56 (81%) | n= 2 (3%) |
| Blood in urine | n=66 (96%) | n=0 (0%) |
| Urinary urgency | n=42 (61%) | n= 14 (20%) |
| Urinary incontinence | n=56 (81%) | n=5 (7%) |
| Achieve and maintain erection | n=47 (68%) | n=17 (25%) |
| Ejaculation | n= 46 (67%) | n=18 (26%) |
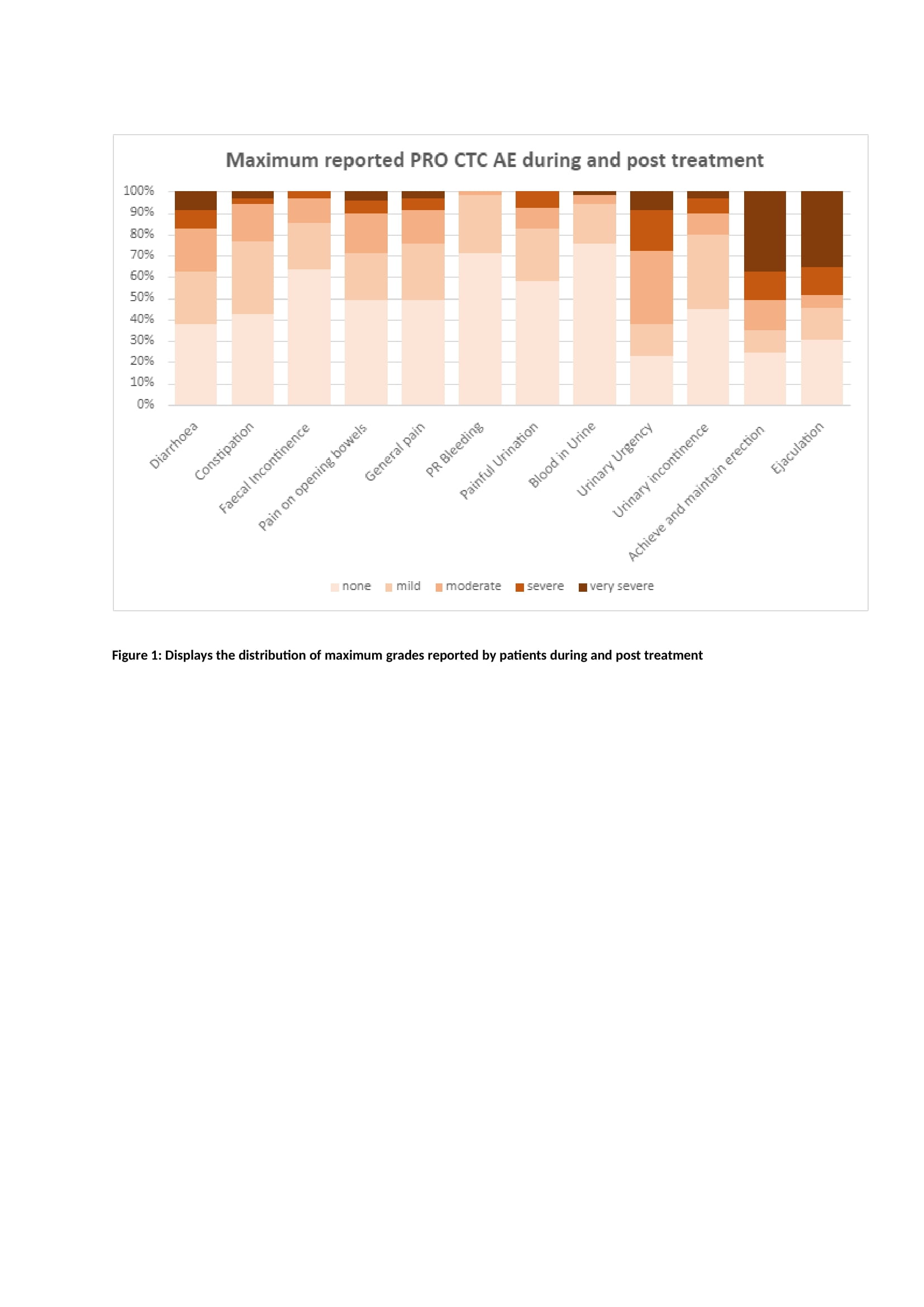
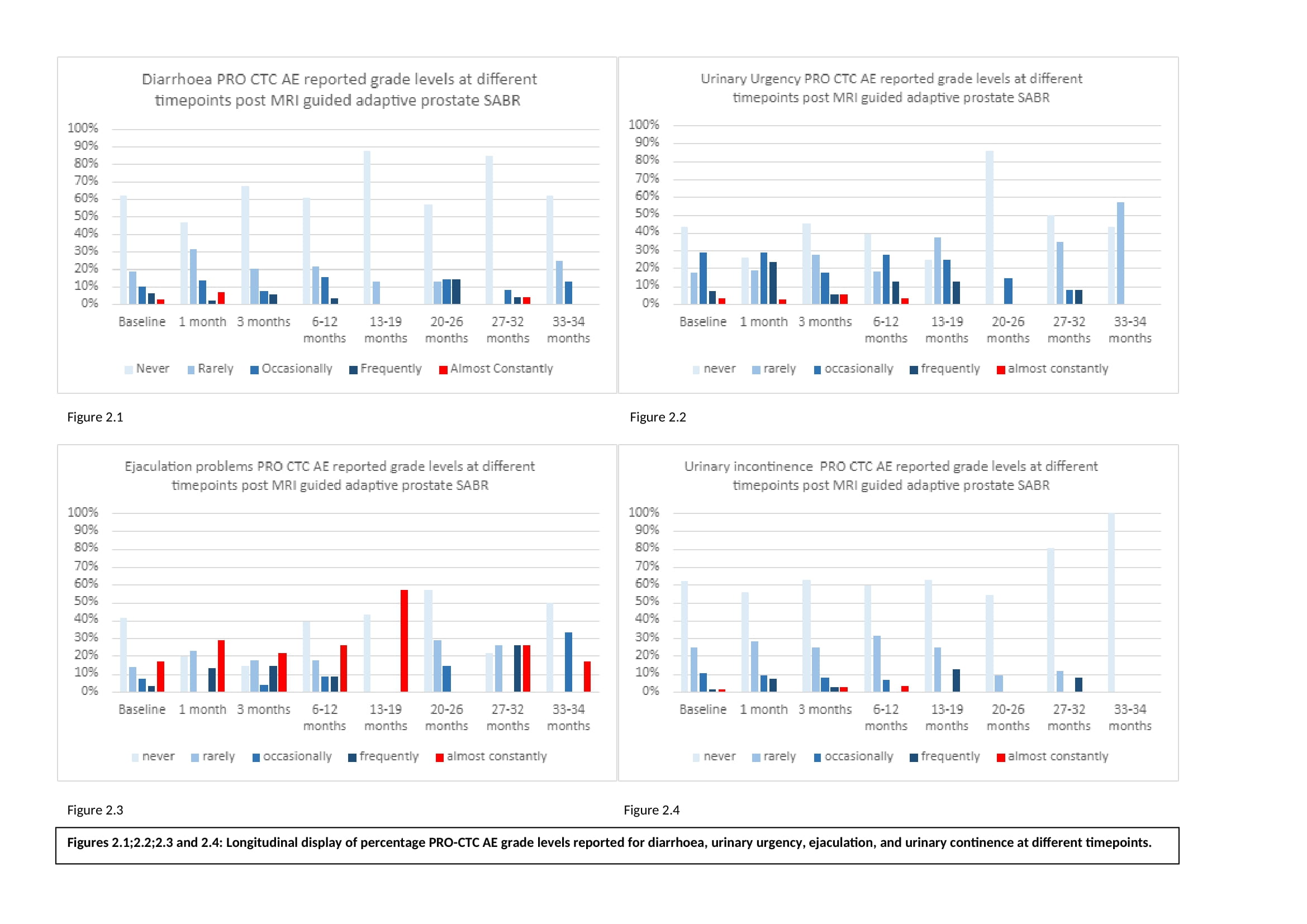
Conclusion
Our study is, to date, the largest patient reported outcomes study post MRI guided daily adaptive prostate SABR. It shows acceptable levels of toxicity up to 34 months post treatment