Prospective trial evaluating a novel MR-based 3D-printed head immobilization device (NCT04114786)
PD-0654
Abstract
Prospective trial evaluating a novel MR-based 3D-printed head immobilization device (NCT04114786)
Authors: Paola Jablonska1, Nancy LaMacchia2, Amy Parent2, Harley Chan3, Matthew Filleti3, Matthew Ramotar2, Young-Bin Cho4, Anna Santiago5, Maria Braganza2, Adam Bandzynski6, Normand Laperriere7, David Shultz7, Tatiana Conrad7, Derek Tsang7, Barbara-Ann Millar7, Tony Tadic7, Alejandro Berlin7
1Clinica Universidad de Navarra, Radiation Oncology, Pamplona, Spain; 2Princess Margaret Cancer Centre, Radiation Medicine Program, Toronto, Canada; 3University Health Network, Radiation Physics, Toronto, Canada; 4Cleveland Clinic, Radiation Oncology, Ohio, USA; 5Princess Margaret Cancer Centre, Biostatistics, Toronto, Canada; 6Princess Margaret Cancer Centre, Cancer Digital Intelligence Program, Toronto, Canada; 7Princess Margaret Cancer Centre, Radiation Oncology, Toronto, Canada
Show Affiliations
Hide Affiliations
Purpose or Objective
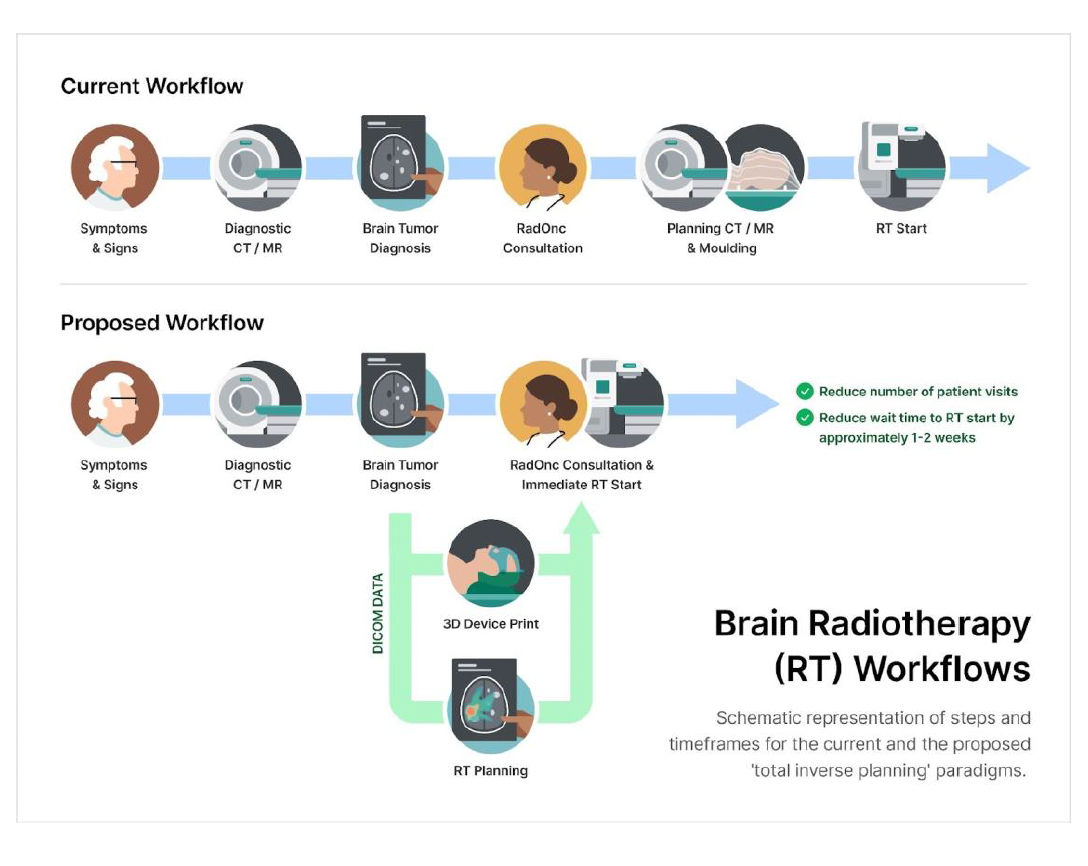
Immobilization and reproducible positioning are crucial for accurate brain radiotherapy (RT). Current RT planning processes require redundant dedicated imaging studies and a bespoke moulding session to create an immobilization device (i.e., thermoplastic mask [T-mask]). Innovative approaches may improve the patient’s journey and value of care. The aim of this study was to prospectively deploy and assess the performance of a patient-specific 3D-printed mask (3Dp-mask) that is generated solely from MR imaging, allowing for the recreation of a reproducible tolerable positioning and immobilization for patients undergoing brain RT.
Material and Methods
Patients undergoing LINAC-based CNS RT (primary brain tumor or resected brain metastases) were enrolled prospectively (IRB #18-5753; NCT04114786). In the investigational arm, an in-house designed 3Dp-mask was generated from MR images to recreate the natural random head positioning during MR acquisition and allow coupling with the LINAC table during RT delivery. Differences in inter-fraction motion were compared between patients treated in the control (T-mask) versus the investigational (3Dp-mask) paradigm. Adverse events and tolerability were assessed using a patient-reported questionnaire after conventional moulding session, and by the end of the first and last weeks of treatment for both arms.
Results
Between January 2020 and July 2022, a total of 40 patients were enrolled (20 on each arm). All participants completed the prescribed brain RT and all study evaluations. Median time from simulation to ready-to-treatment was 4 (range 1 -12) and 8 days (range 6 -14) for the control and investigational arms, respectively. The average time to complete the 3Dp-mask design and printing was 36h 50min (range 12h 56min - 42h 01min). There were no significant differences in the ratings between groups through each questionnaire time point, except for a greater reduction in neck discomfort in the investigational arm compared to the control arm from end of first RT week to end of last RT week (B= -0.28, CI= [-0.49, -0.06], p=.011). Both arms showed similar shift values for the left, right, posterior, superior, and inferior. There was a larger absolute anterior-posterior displacement in the control arm than in the investigational arm (mean [sd] 0.210cm [0.126] verus 0.096cm [0.076], p=.001). Absolute superior-inferior displacement tended to be larger in the investigational arm (mean [sd] 0.197cm [0.137] versus 0.103c, [0.075] in the control arm, p=.01).
Conclusion
The proposed total inverse planning paradigm using a 3D-printed immobilization device is feasible, rendering comparable inter-fraction performance while offering a better patient experience compared to the conventional thermoplastic mask. This approach could allow improvements to the brain RT workflows and potentially significant cost savings.