Outcomes of stereotactic radiosurgery for colorectal cancer brain metastases
PD-0646
Abstract
Outcomes of stereotactic radiosurgery for colorectal cancer brain metastases
Authors: Chengcheng Gui1, Kirin D. Mueller1, Jordan E. Eichholz2, Henry Walch2, Ishaani Khatri1, Luke del Balzo1, Nancy E. Kemeny3, Brandon S. Imber1, Nikolaus Schultz4, Michael B. Foote3, Rona D. Yaeger3, Luke R. G. Pike1
1Memorial Sloan Kettering Cancer Center, Department of Radiation Oncology, New York, New York, USA; 2Memorial Sloan Kettering Cancer Center, Sloan Kettering Institute, New York, New York, USA; 3Memorial Sloan Kettering Cancer Center, Department of Medicine, New York, New York, USA; 4Memorial Sloan Kettering Cancer Center, Department of Epidemiology and Biostatistics, New York, New York, USA
Show Affiliations
Hide Affiliations
Purpose or Objective
Brain metastases (BM) from colorectal cancer (CRC) are rarely observed and portend a poor prognosis. Stereotactic radiosurgery (SRS) remains a mainstay in treating CRC BM when the burden of intracranial disease is low. This study evaluated the oncologic outcomes and prognostic factors after SRS for CRC BM.
Material and Methods
Patients with newly diagnosed CRC BM treated with SRS alone or postoperative SRS to the resection cavity at a single institution between 2009 and 2022 were included. Patients who received whole-brain radiation therapy as part of initial treatment were excluded. To enable forthcoming genomic analyses, this cohort was limited to patients with genomic profiling of at least one intracranial or extracranial tumor.
Overall survival (OS), intracranial progression-free survival (iPFS), local recurrence (LR), and baseline characteristics of the patient, disease, and SRS were obtained from clinical notes and contrast-enhanced MRI brain. Kaplan-Meier modeling, logistic regression, and Cox regression were used in the analysis. Genomic profiling was performed with one of four versions (341, 410, 468, or 505-gene) of MSK-IMPACT, a custom FDA-approved hybrid-capture sequencing assay.
Results
This study included 124 patients with 304 CRC BM. Sixty-one patients (49%) had rectal cancer. At BM diagnosis, the median age was 56 years (range 25-91). The median number of BM per patient and median BM size were 2 (range 1-16) and 1.1 cm (range 0.2-5.5), respectively. Sixty-six BM (22%) received postoperative SRS. The median prescription was 27 Gy (range 16-30) in 3 fractions (range 1-5), and the median biologically effective dose (BED, α/β = 10) was 51.3 Gy (range 35.7-73.3).
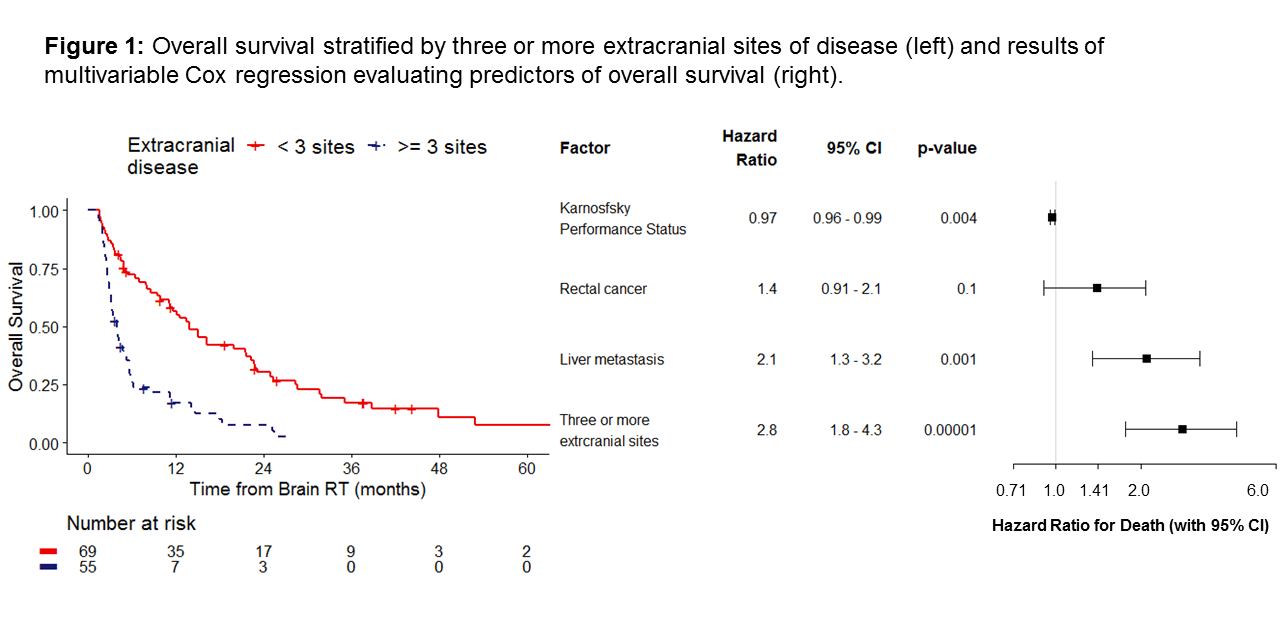
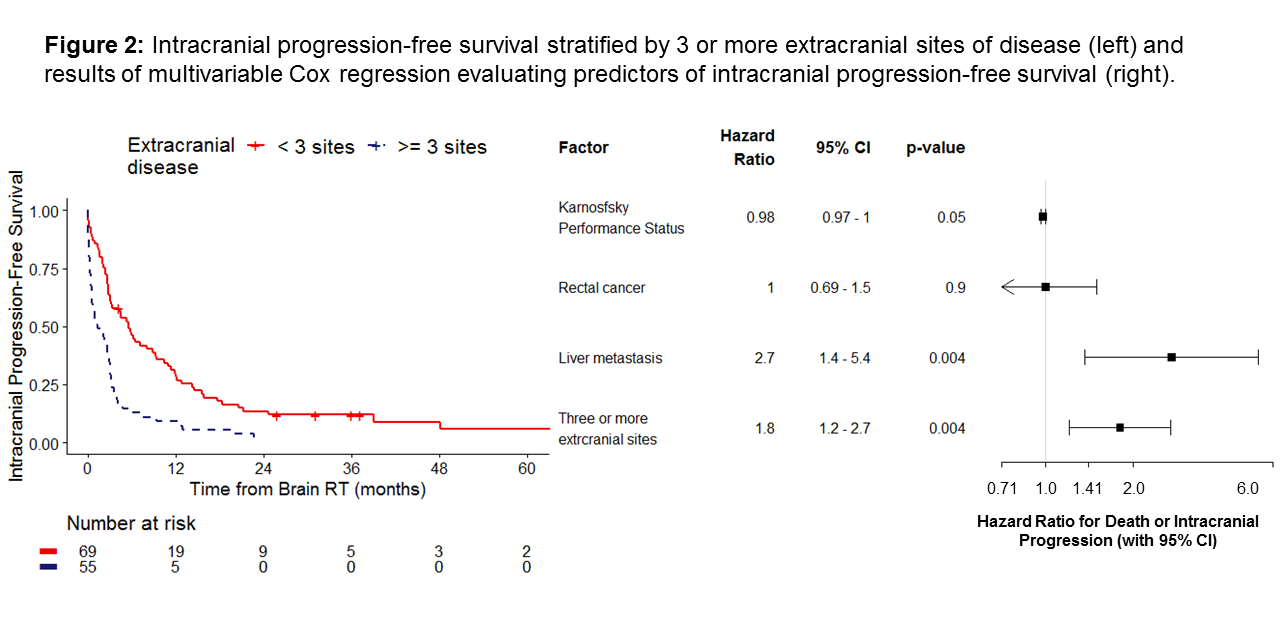
Median OS and iPFS were 7 months (95% CI 5.0-12) and 3 months (95% CI 2.5-3.9), respectively. On multivariable Cox regression, OS was longer in patients with better Karnofksy Performance Status (HR=0.97, p=0.004) and worse in patients with liver metastases (HR=2.1, p=0.001) and ≥3 sites of extracranial disease (HR=2.8, p<0.0001). These factors were also associated with iPFS (p<0.05). Intracranial progression alone was associated with the initial number of BM (HR=1.1, p=0.01) and progressive extracranial disease (HR=2.65, p=0.01).
There were 20 cases of LR at median 2.8 months. On univariable logistic regression, greater PTV volume and BED < 51.3 Gy were associated with increased LR at 6 months. On multivariable regression, only BED < 51.3 Gy was significant (OR=4.5, p=0.02). All patients had at least one sample for MSK-IMPACT analysis. Associations between genomic features and CNS-specific outcomes are forthcoming.
Conclusion
We present a large cohort of patients with CRC BM treated with SRS. Uncontrolled extracranial disease at the time of BM diagnosis was associated with both poorer OS and higher risk of intracranial progression. Higher BED was correlated with better local control, independent of tumor size. Future analyses will explore the genomic features that may further explain differences in oncologic outcomes.