Adaptive aperture optimization in proton therapy to improve NTCP in head and neck cancer patients
PO-1983
Abstract
Adaptive aperture optimization in proton therapy to improve NTCP in head and neck cancer patients
Authors: Marta Bogowicz1, Vicki Trier Taasti1, Mirko Unipan1, Geert Bosmans1, Wouter van Elmpt1
1Department of Radiation Oncology (Maastro), GROW School for Oncology, Maastricht University Medical Centre+, Maastricht, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
The inclusion of an aperture in spot scanning proton beam delivery systems was shown to improve lateral dose fall-off. However, in most studies either only one aperture per beam or one per energy layer was used, which allows to trim only spots on the edge of the spot map. Trimming of the inner spots could be beneficial in systems with relatively big spots. In this study, we investigated the optimal number of apertures per energy layer to improve normal tissue complication probability (NTCP) in head and neck (HN) cancer patients.
Material and Methods
Four patients with HN cancer were included in this analysis. The original clinical treatment plans were created in RayStation v11A for a Mevion Hyperscan S250i proton beam delivery system. This system is equipped with a proton MLC called adaptive aperture (AA), which allows for dynamic collimation of the proton beam depending on target outline at given radiological depth, one AA shape per energy layer. A script was made to modify the original plan by adding extra AA shapes at every energy layer. Three additional plans were made to test the optimal number of AA shapes in order to obtain the largest dosimetric benefit with the lowest plan complexity. The new AA shapes were created by shifting the original AA towards the center of the spot map. Plans with 1, 2 or 3 extra AA shapes were made, with the new AA shapes following closely the 1st-, 2nd- and 3rd-outermost contour on the spot map (Fig. 1). The spot weights were further optimized in RayStation, objectives were adjusted if needed. The new plans were robustly evaluated (3% range and 3mm setup uncertainty). The dosimetric benefit was assessed based on the CTV coverage and NTCP reduction for grade 2 (G2) and 3 (G3) dysphagia and xerostomia. For the AA configuration with the largest dosimetric benefit plan QA using Octavius (PTW, Germany) was performed. Beam delivery time was noted.
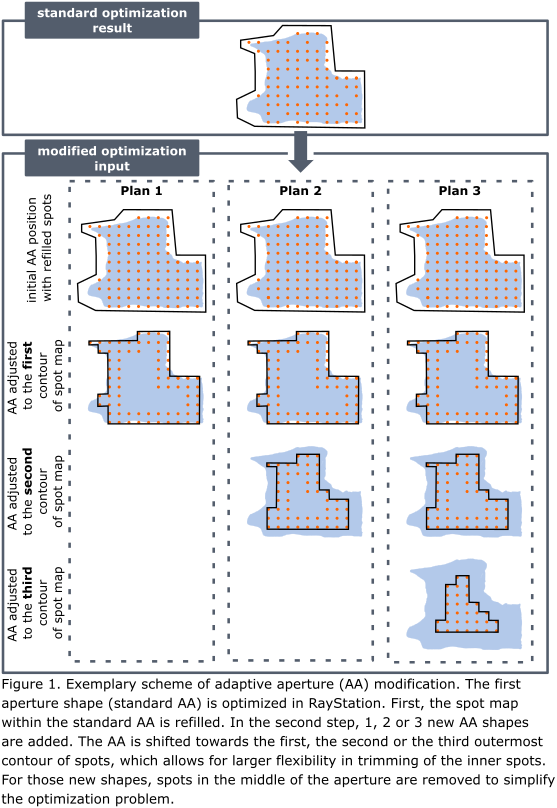
Results
In all cases, CTV coverage either improved or remained above the constraint. In most plans, the NTCP was reduced (Fig. 2). For patient 3 only a small improvement was observed. In this case, the high dose CTV was located more caudally than for others and thus for the most of the relevant organs at risk a low dose was delivered in the original plan. The highest dosimetric improvement was observed for the plan with 2 extra AA shapes. For patient 1, a reduction of mean dose in the pharyngeal constrictor muscle (PCM) inferior = 3Gy, PCM middle = 3Gy and oral cavity = 2Gy led to ΔNTCP=3.4% in G2 dysphagia. The new plans passed the plan QA (gamma 3%/3mm > 95%). Delivery time increased by around 65% in comparison to the original plan (from approx. 5.5 min to approx. 9 min).
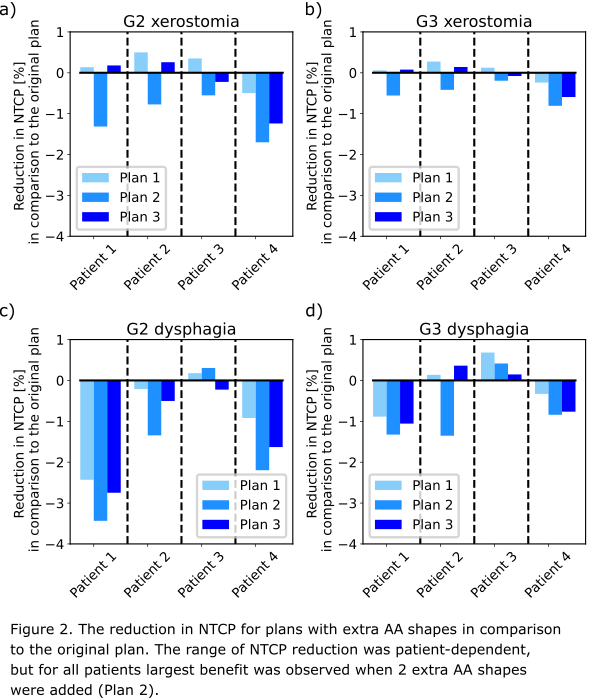
Conclusion
This study presented a proof of concept describing the optimization of aperture positions for HN cancer. Proton plans with multiple AA shapes have longer delivery times, but this is acceptable giving the dosimetric benefit. Forward optimization of AA shapes resulted in clinically relevant NTCP reduction with preserved target coverage.