Cochlear-optimized plan comparison between protons vs. photon radiosurgery for vestibular schwannoma
PO-1958
Abstract
Cochlear-optimized plan comparison between protons vs. photon radiosurgery for vestibular schwannoma
Authors: Kimberley S. Koetsier1,2, Michelle Oud2, Erik de Klerck2, Erik F. Hensen1, Marco van Vulpen3, Anne van Linge4, Cleo Slagter2, Steven Habraken2,5, Mischa S. Hoogeman2,5, Alejandra Méndez Romero2,6
1Leiden University Medical Center, Department of Otorhinolaryngology and Head and Neck Surgery, Leiden, The Netherlands; 2Erasmus MC Cancer Institute, University Medical Center Rotterdam, Department of Radiotherapy, Rotterdam, The Netherlands; 3HollandPTC, Department of Radiotherapy, Delft, The Netherlands; 4Erasmus MC, Department of Otorhinolaryngology and Head and Neck Surgery, Rotterdam, The Netherlands; 5HollandPTC, Department of Medical Physics & Informatics, Delft, The Netherlands; 6HollandPTC, Department of Radiotherapy, Rotterdam, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Cochlear doses have been related to hearing loss after radiosurgery for patients with vestibular schwannoma. The objective of this study was to compare the reduction of cochlear dose in robotic photon radiosurgery and intensity modulated proton therapy (IMPT) treatment planning for vestibular schwannomas.
Material and Methods
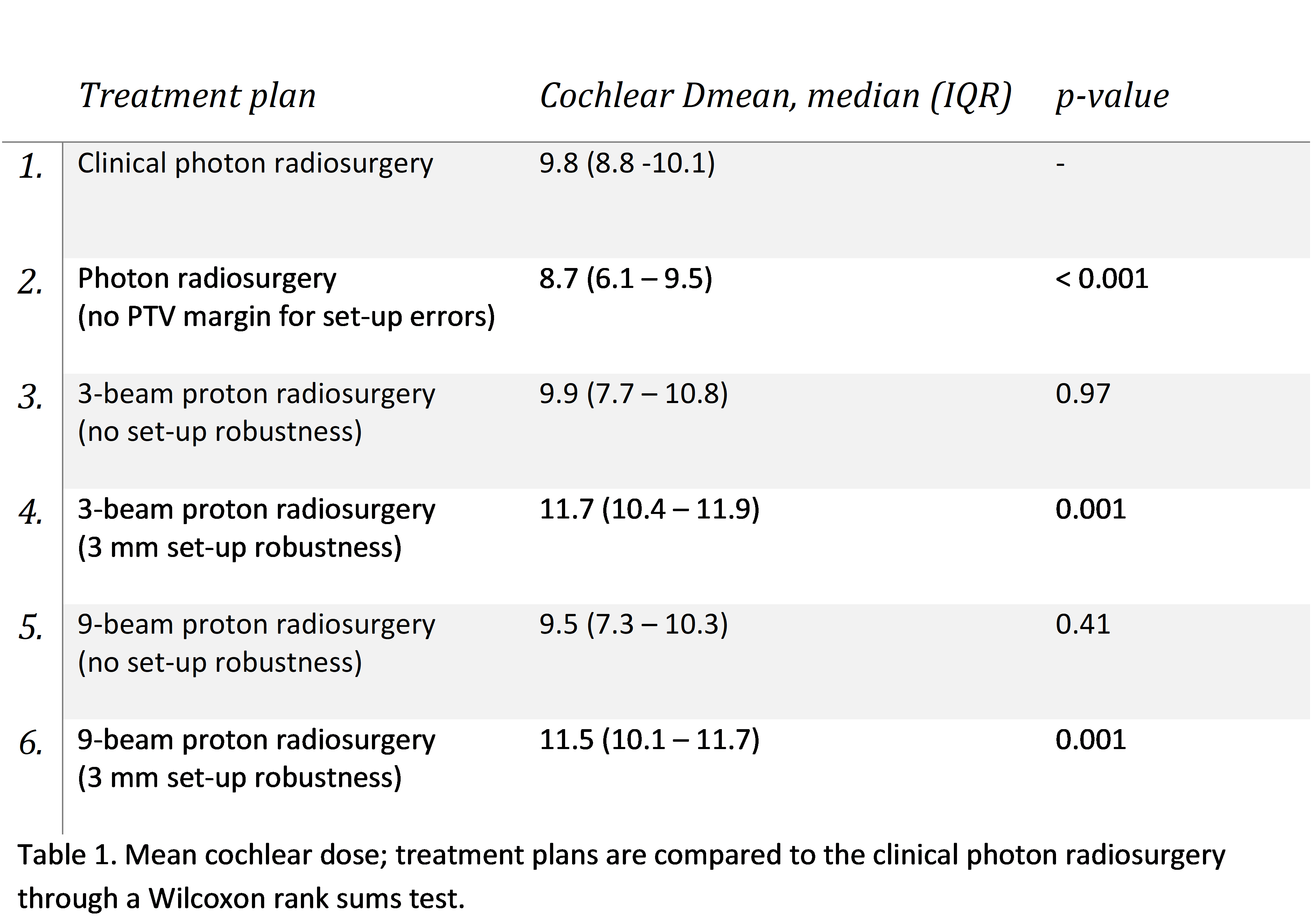
Delivered treatment plans with robotic photon radiosurgery that were not optimized on cochlear dose were compared to five cochlear-optimized treatment plans: one photon plan and four proton plans (total of 120 plans). A dose of 12 Gy was prescribed at the 80% isodose line. As in clinical practice, photon plans were generated without adding a PTV margin for set-up errors. IMPT plans were generated using 3 or 9 beams and applying no robustness for set-up errors (only 3% range uncertainty) or through including 3 mm set-up robustness during plan optimization and evaluation. All plans needed to meet the clinical constraints including 98% tumor coverage. Proton plans were generated with an in-house system for automated multi-criterial treatment planning and photon plans with Precision (Cyberknife, Accuray). Primary outcome was the mean cochlear dose (Dmean). Secondary outcomes included Paddick’s conformity index (PCI) and dose to the organs at risk (OAR). The calculated sample was 24 patients, which was based on reducing the cochlear Dmean >1.5 Gy compared to the clinical plans.
Results
In 11 out of 24 patients, cochlear-optimized photon radiosurgery plans improved the cochlear Dmean >1.5 Gy (mean improvement 2.5 Gy; average cochlear Dmean 5.7 Gy) compared to the clinically delivered plans. In four patients this could be achieved with 3-beam proton plans without set-up robustness (mean improvement 2.1 Gy; average Dmean 5.1 Gy) and in six patients using a 9-beam proton plans without set-up robustness (mean improvement 2.1 Gy; average Dmean 5.5 Gy). When the 3 mm set-up robustness was added, none of the plans showed an improvement >1.5 Gy. Tumors with a larger distance to the cochlea showed the most cochlear sparing. PCI were comparable between the photon plans (1.2), the 9-beam proton plans (1.2) and the 3-beam proton plan (1.3) without set-up robustness. Adding 3 mm set-up robustness to the proton plans increased the PCI to 2.6 and 2.3. Photon plans provided lower brainstem Dnear-max(35mm3) vs. protons (11.9 vs 12.1 (no set-up robustness); 12.5 Gy (with set-up robustness), while all proton plans resulted in a lower brainstem Dmedian. Using nine proton beams (instead of three) resulted in reduced dose to surrounding tissue.
Conclusion
Cochlear-optimized photon robotic radiosurgery plans provided better cochlea sparing than proton plans in vestibular schwannoma patients. Proton radiotherapy may provide similar cochlea sparing and reduced dose to other OARs without setup-up robustness. Proton radiotherapy is capable of providing lower dose to OARs that are distant to the tumor, but not for the organs directly adjacent to it.