Feasibility of bone marrow sparing VMAT and Dixon MRI for the PROTECT study
Anouk Corbeau,
The Netherlands
PO-1422
Abstract
Feasibility of bone marrow sparing VMAT and Dixon MRI for the PROTECT study
Authors: Anouk Corbeau1, Remi Nout2, Sander Kuipers2,3, Eleftheria Astreinidou1, Jan Willem Mens2, Ernst Harderwijk1, Birgit Nielen1, Piotr Wielopolski4, Abdul Sharfo2, Nanda Horeweg1, Jeremy Godart2,5, Uulke van der Heide1, Carien Creutzberg1, Stephanie de Boer1
1Leiden University Medical Center, Department of Radiation Oncology, Leiden, The Netherlands; 2Erasmus MC Cancer Institute, University Medical Center Rotterdam, Department of Radiotherapy, Rotterdam, The Netherlands; 3HollandPTC, Department of Medical Physics and Informatics, Deflt, The Netherlands; 4Erasmus MC, University Medical Center Rotterdam, Department of Radiology and Nuclear Medicine, Rotterdam, The Netherlands; 5HollandPTC, Department of Medical Physics and Informatics, Delft, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Women undergoing concurrent radio(chemo)therapy and brachytherapy for locally advanced cervical cancer (LACC) often experience treatment-related morbidity, including hematologic toxicity resulting from bone marrow (BM) suppression. Both proton therapy and BM sparing (BMS) radiotherapy could reduce dose to BM. The prospective, multi-center PROTECT study investigates the difference between BMS intensity modulated proton therapy (IMPT) and IMRT/VMAT photon therapy in clinical practice. BM activity, defined as BM fat, will be evaluated with MR scans with the Dixon sequence, as well as by serial blood sampling. However, BMS radiotherapy was not yet in clinical use among the participating centers and in clinical practice a breath-hold Dixon sequence is only applied for liver fat quantification. The aim of this project was to implement BMS treatment planning and Dixon MR scanning for BM quantification in the clinic.
Material and Methods
The PROTECT study is registered with clinicaltrials.gov (NCT05406856) and the study protocol has been previously published. The PROTECT study consists of two sequential phases in which both fifteen women with LACC will be enrolled. In phase I, participants are treated with BMS IMRT/VMAT, in phase II with BMS IMPT. For the BMS radiotherapy, a systematic literature review on delineation methods for BM as OAR and predictive dose volume parameters for hematologic toxicity was performed. Soft dose constraints were identified and tested by retrospectively generating treatment plans for three to four patients. With respect to the Dixon MRI, the standard exam card was evaluated for vertebral BM fat quantification in multiple volunteers and optimized per field strength and scanner.
Results
Phase I of the PROTECT study is now ongoing and five participants were included for the IMRT/VMAT chemoradiation. The soft dose constraints tested and used for BMS are V10<90%, V20<65%, and V40<15-20%. Figure 1 provides an example of a treatment plan with and without BMS. Both the BMS constraints and the EMBRACE-II target and OAR constraints were met in all five participants. For the Dixon MRI, both the quality and clinical feasibility of the measurements had to be balanced. In order to enable scanning resolution improvement, a free-breathing protocol was applied. Consequent breathing artifacts could be reduced by changing the phase encoding direction. Figure 2 gives an example of the Dixon MR scans.
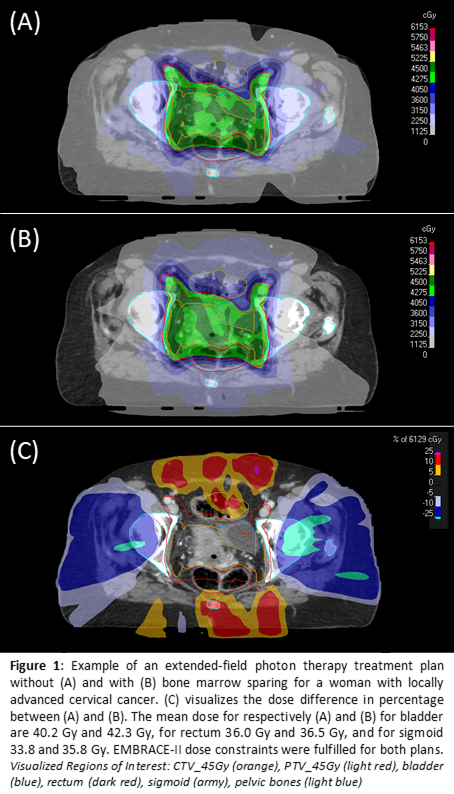
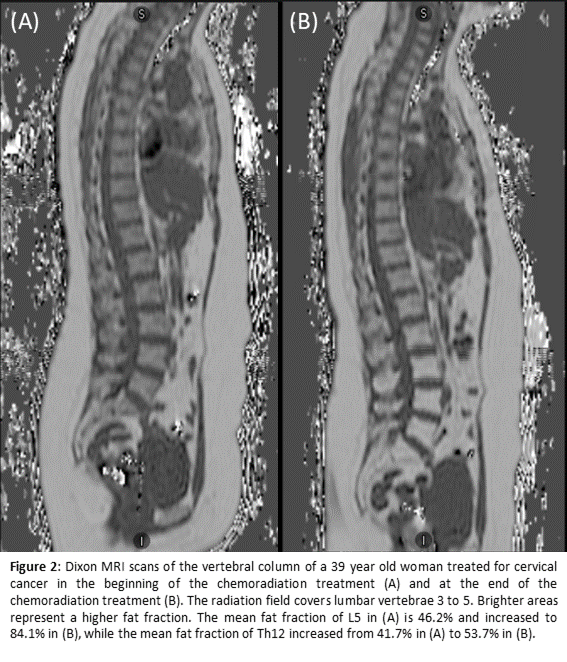
Conclusion
The introduction of BMS treatment planning and the Dixon MRI for BM fat quantification in the clinic to facilitate the PROTECT study was feasible. BMS treatment planning could be safely implemented while fulfilling the EMBRACE-II dose constraints. Dixon MR scans combined with serial blood sampling will facilitate immune response evaluation. Further inclusion of patients in this study will help to quantify the potential clinical gain (and costs) of BMS IMPT for LACC.