Development of a µCT radiomic platform to identify radio-immune signatures in murine tumor models
PO-2243
Abstract
Development of a µCT radiomic platform to identify radio-immune signatures in murine tumor models
Authors: Giulia Mazzaschi1, Morgane Dos Santos2, Paul Bergeron1, Lisa Sitterle1, Marine Gerbé De Thoré1, Winchygn Liu1, Céline Clémenson1, Lydia Meziani1, Alessandra Gonnelli1, Olivia Bawa3, Amaury Leroy1, Roger Sun1, Theophraste Henry1, Pierre-Antoine Laurent1, Lucas Moron Dalla Tor4, Federico Quaini4, Marcello Tiseo5, Fabien Milliat2, Charlotte Robert1, Michele Mondini1, Eric Deutsch6
1Gustave Roussy, INSERM U1030, Paris, France; 2Institute for Radiological Protection and Nuclear Safety (IRSN), Radiobiology and regenerative Medicine (SERAMED), Laboratory of Medical Radiobiology (LRMed), Paris, France; 3Gustave Roussy, Plateforme de pathologie expérimentale et translationnelle, UMS AMMICA, Paris, France; 4University of Parma, Medicine and Surgery, Parma, Italy; 5University Hospital of Parma, Medicine and Surgery, Medical Oncology Unit, Parma, Italy; 6Gustave Roussy, INSERM U1030 and Department of Radiation Oncology, Paris, France
Show Affiliations
Hide Affiliations
Purpose or Objective
The ground-breaking results of immunotherapy (IT) in cancer patients still leave uncovered multiple drawbacks on the current clinical translation of cancer-immune interplay.By converting imaging data into high-throughput features, radiomics emerged as a non-invasive tool to decode tumor immune microenvironment (TIME) and predict IT response. Nonetheless, lack of standardization and methodological issues limit its clinical application. Scarce radiomic data in preclinical tumor settings have been generated so far.
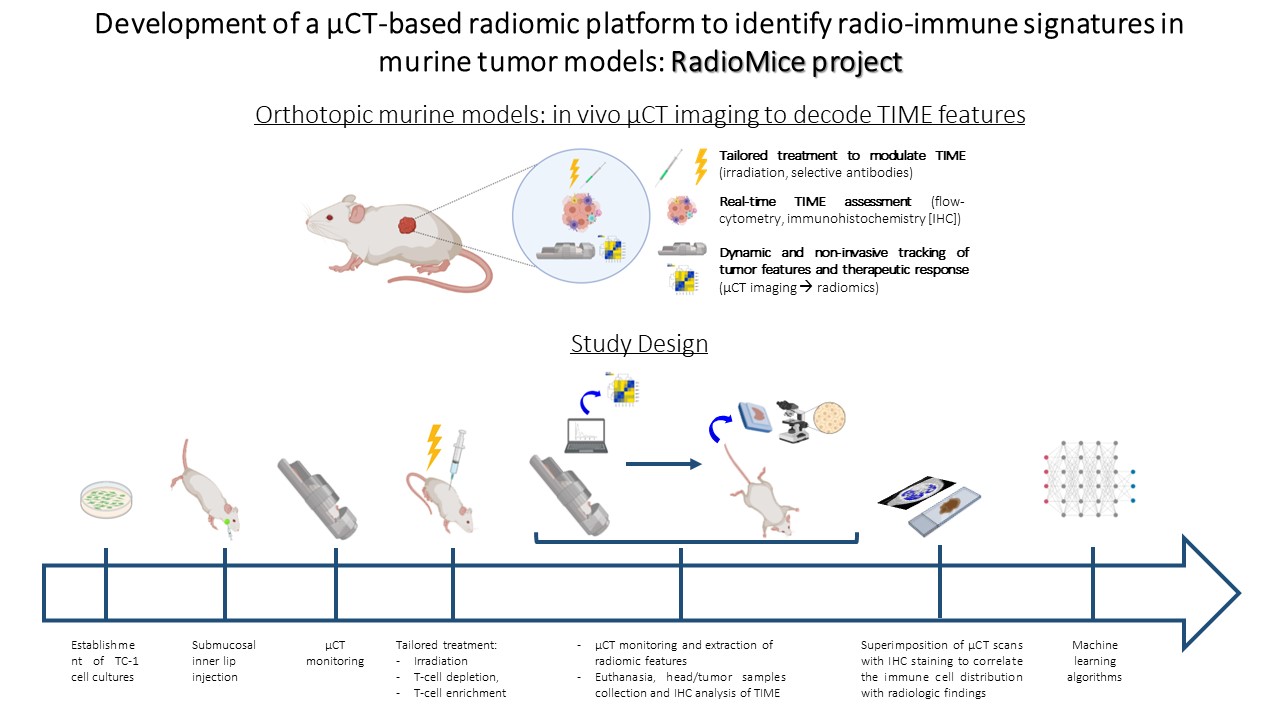
The aim of our study was to develop a µCT-based radiomic platform in orthotopic murine models of head and neck cancer, to ultimately validate the biological reality of a radiomic approach in the evaluation of tumor infiltrating lymphocytes (TILs).
Material and Methods
We established orthotopic models of head and neck cancer in immunocompetent mice. Tailored regimens including radiotherapy + selective antibodies were employed to modulate TIME (T cell enrichment vs depletion). In vivo preclinical imaging was performed using the Quantum FX µCT technology; µCT scans were processed for radiomic features (RFs) extraction (pyradiomics). Mice heads (comprising tumors) were collected and subjected to decalcification/inclusion protocol. Representative 5µm sections of the entire tumor area were cut from tissue block at constant distance of 500µm and immunohistochemically (IHC) stained for CD8 marker. A dedicated reconstruction algorithm was employed to generate a 3D map of CD8+ TILs (Figure 1).
Results
1. Development and optimization of a quantitative µCT imaging approach: we selected the best acquisition and reconstruction protocol able to minimize noise, improve spatial resolution and overcome potential miscalibration errors, thereby demonstrating the feasibility and reproducibility of µCT imaging in murine models.
2. Deployment of a reproducible radiomic pipeline: after image pre-processing, including voxel resampling and image discretization, we delineated the volumes of interest (VOI) outlining the entire tumor region, which acted as source of information for quantitative analyses at tumor scale. Overall, 106 µCT based RFs (shape, first- and second- order) were extracted and correlated with distinct TIME.
3. Registration of reconstructed IHC images over µCT scans: we obtained a 3D map of CD8+ TILs, which was subsequently readjusted to the anatomical volume obtained by µCT. IHC images were superimposed over µCT scans to correlate the spatial distribution of immune cells with µCT textural parameters.
Conclusion
We generated a reproducible µCT radiomic platform able to non-invasively decipher TIME features in murine models. Exploiting, in finely controlled laboratory settings, the potential of radiomics to timely assess distinct immune parameters and predict treatment outcome, may partly overcome the limitations observed in clinical practice and implement the personalization of therapeutic strategies.