Proposal for Cyberknife Monte Carlo calculation algorithm model validation
CELINE KARLA TORZSOK,
Chile
PO-1813
Abstract
Proposal for Cyberknife Monte Carlo calculation algorithm model validation
Authors: CELINE KARLA TORZSOK1, Alvaro Ruiz2, Marcelo Ribeiro2, Filippo Marangoni3, Jhonalbert Aponte2, Rixy Plata2, Matias Pino2, Jose Luis Rodriguez4, Herve Broque2
1Falp Fundacion Arturo Lopez Perez, radiation therapy, Santiago, Chile; 2Falp Fundacion Arturo Lopez Perez, Radiation Therapy, Santiago, Chile; 3Falp Fundacion Arturo Lopez Perez, Radiation Therapy, Radiation Therapy, Santiago, Chile; 4Falp Fundacion Arturo Lopez Perez,, Radiation Therapy, Santiago, Chile
Show Affiliations
Hide Affiliations
Purpose or Objective
MC is considered the most accurate algorithm when heterogeneity calculations are needed. There are no specific reports addressing dosimetric validations prior to clinical implementations of MC using CK. Some TPS manufacturers recommend post-modeling tests for Linacs model validation. In our institution, we elaborated a Precision TPS CK-MC procedure including and adapting MPPG5.a recommendations. Seeing how we struggled looking for CK specific reference fields, this work aims to present a validation script proposal.
Material and Methods
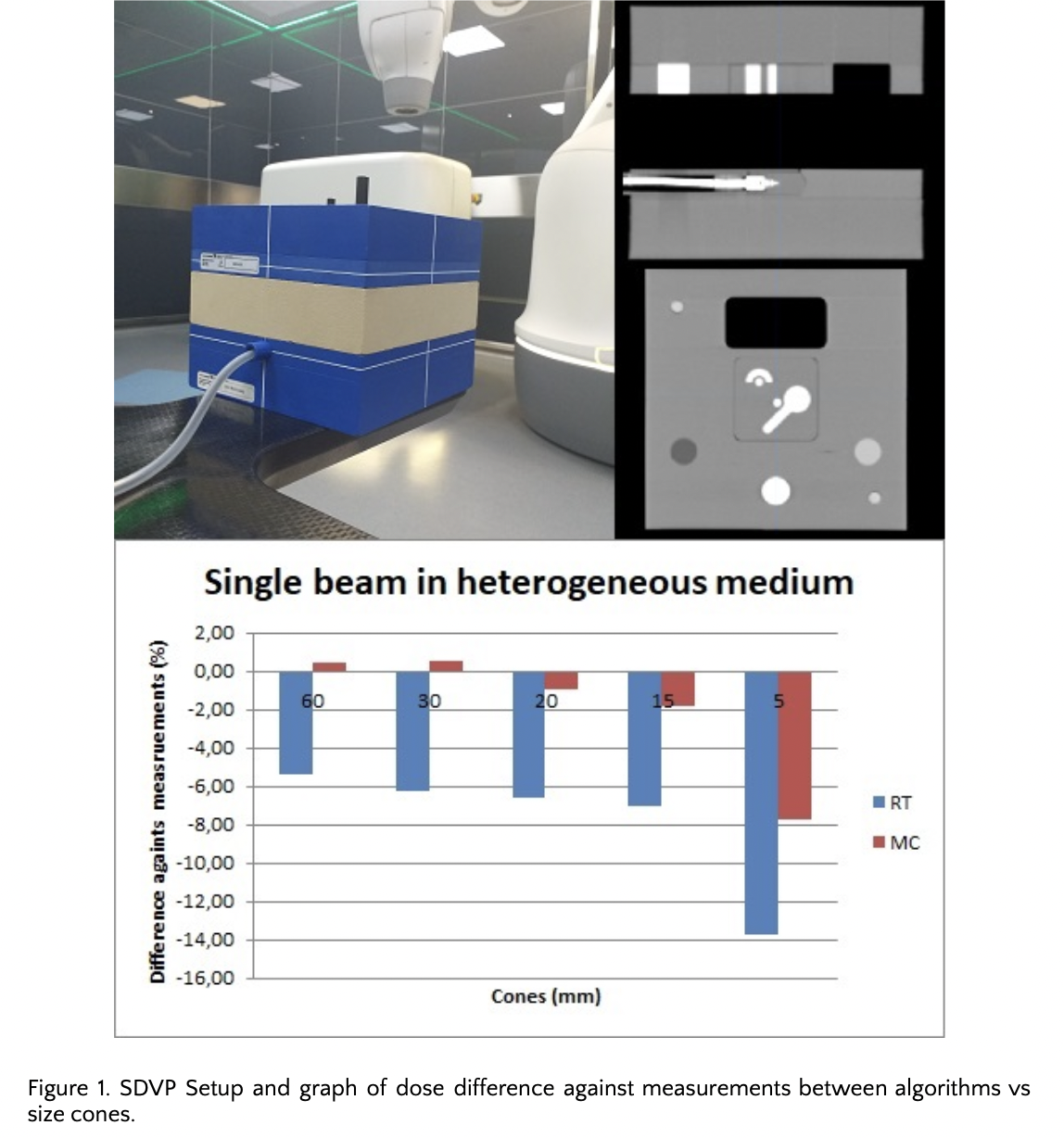
The iterative MC manufacturer workflow was followed until an acceptable match was achieved for profiles, TPR and OF. To validate the model, a Stereotactic Dose Verification Phantom (SDVP), with different density interfaces (air, lung, bone, solid water and high density material) was used. Five different configurations allowed us to create plans, compare and measure MC single and complex beam sets and their equivalent Ray Tracing (RT) calculations. Additionally, patient specific QAs were elaborated. Measurements, using IBA-CC01 ionization chamber and EBT3 films, were performed to evaluate point dose and dose distribution.
Results
Comparisons for single beam calculations had maximum differences between MC and point dose measurements in respectively heterogeneous and homogeneous media of -7.7% and -6.8%. In real patient cases, the maximum difference found was -6.0%. In general, when the cone was smaller, the difference against measurement was higher, and in comparison with RT, MC had the best results (figure 1).
For heterogeneous media, when comparing TPS dose plans with both algorithms: maximum hotspot and gamma pass rate showed that the change due to decreasing the MC uncertainty (from 1 to 0.1%) were respectively -15% to -18% and 77% to 72%. For homogeneous media, they went from +8.1% to 1.8% and 86.6% to 100%.
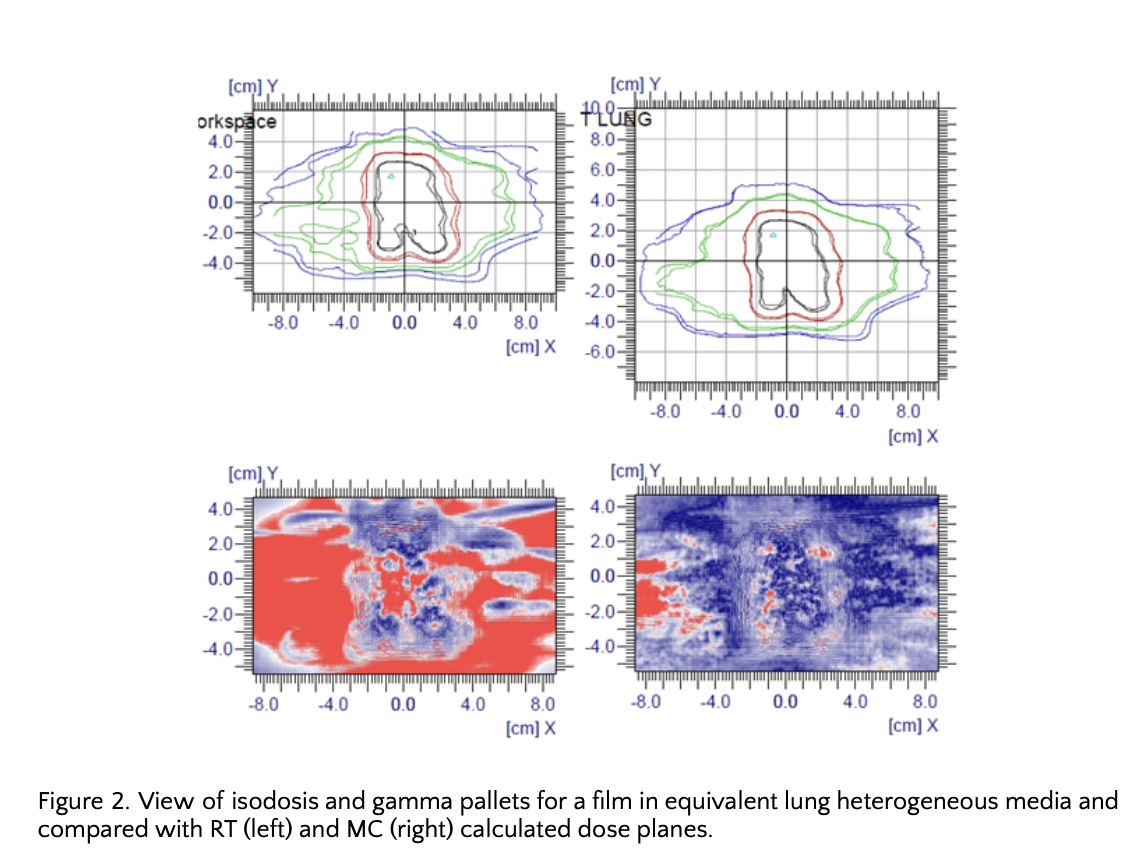
Film analysis in homogeneous media, with RT and MC dose planes, gave practically identical results (98.6% 2%-2mm dose-position gamma pass rate). While, when the media was changed to heterogeneous, it brought quite different gamma analysis results. Using the same parameters and the same ROI, for RT gamma pass rate was 41.82% while for clinical 1% uncertainty MC was 93.07% (figure 2).
Conclusion
We found consistent results with the uncertainty associated with small field sizes and phantom setup. Our validation script also demonstrates that the major differences between RT and MC could be observed when a target is totally inserted in lung material, in contrast with no difference for a soft tissue-surrounded target without using small collimators. We demonstrated that calculations in heterogeneous media with the decided clinical uncertainty of 1% gave us acceptable results.