Workflow optimisation for reproducible printing polymer gel containers in 3D
PO-1775
Abstract
Workflow optimisation for reproducible printing polymer gel containers in 3D
Authors: Wibke Johnen1,2, Alina Elter3,2,4, Elaine Schmuck3,2,4, Armin Runz3,2, Gernot Echner3,2
1German Cancer Research Center (DKFZ), Medical Physics in Radiation Oncology , Heidelberg, Germany; 2National Center for Radiation Research in Oncology (NCRO), Heidelberg Institute for Radiation Oncology (HIRO), Heidelberg, Germany; 3German Cancer Research Center (DKFZ), Medical Physics in Radiation Oncology, Heidelberg, Germany; 4University Hospital Heidelberg, Radiation Oncology, Heidelberg, Germany
Show Affiliations
Hide Affiliations
Purpose or Objective
Polymer gels (PG) were shown to be beneficial for the measurement of 3D dose distributions for the simulation of different treatment scenarios in so-called end-to-end-tests (E2ET). For this purpose, PG containers with anthropomorphic structures for the use in complex E2ET–phantoms are required. In Elter et. al [1] it was shown that 3D printing is suitable if certain measures are taken. Since the PG is very sensitive to interfering influences such as oxygen and other impurities, only a few printing materials and techniques have proven to be suitable. In this study, we present the production process of such containers, optimised in regard to reproducibility and simplified handling.
Material and Methods
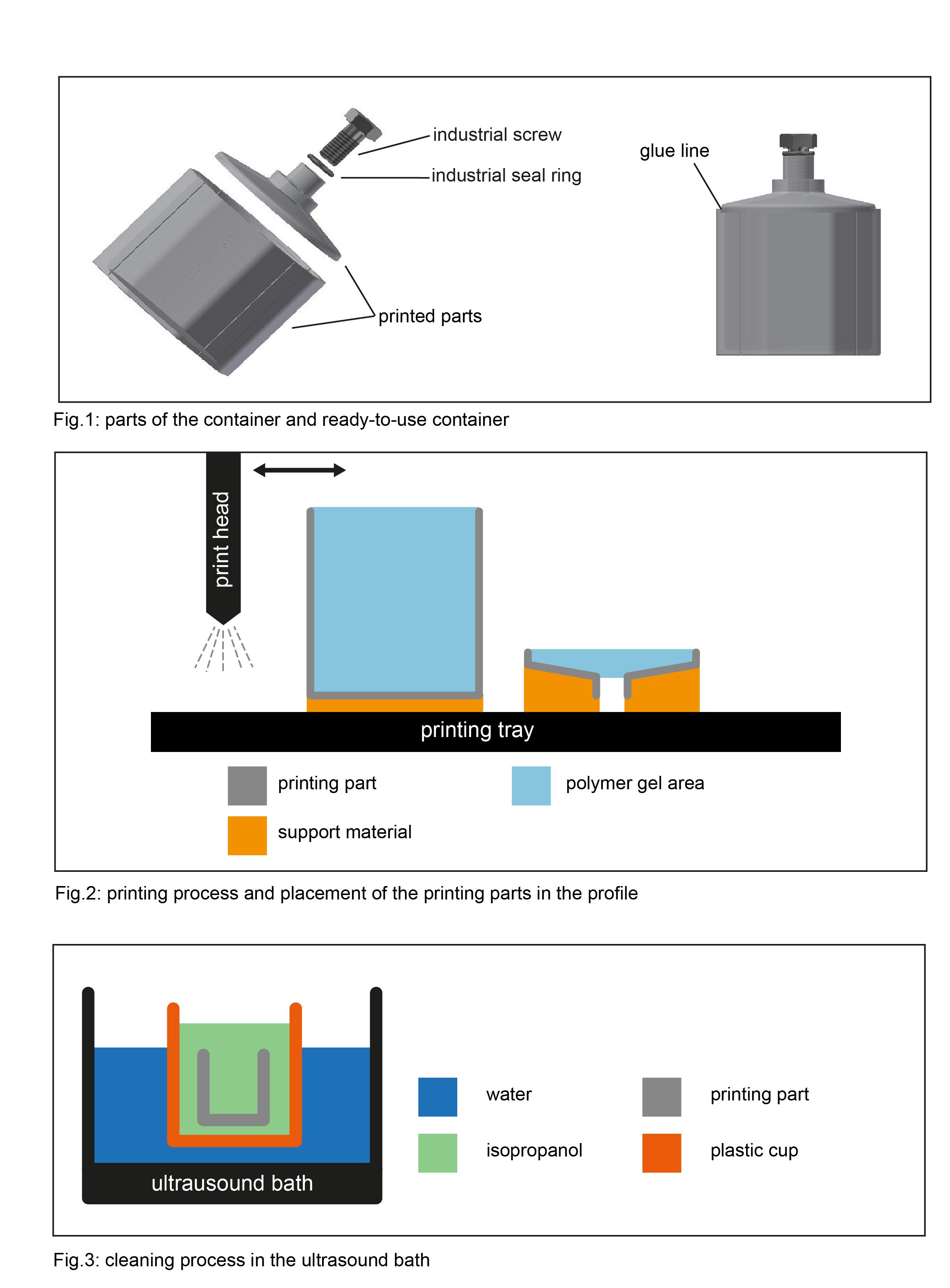
The individual parts of the containers were designed in Inventor 2018 software (Autodesk,USA). The J55^TM Prime and Objet 500 Connex3^TM printers (Stratasys, USA & Israel) were used for production with the PolyJet process using the VeroClear^TM printing material (Stratasys). For final cleaning, the individual parts were soaked in isopropanol and placed in an ultrasound bath (RK 100 H, Bandelin, Berlin) for 30 minutes. For drying and evaporation, the parts were air-dried overnight. After gluing with VeroClear^TM, the containers were filled with PG (Fig.:1). After production, the containers were irradiated with a homogenous dose distribution of 4 Gy using the clinical 6MV MRIdian Linac (ViwRay Inc., USA). Gel evaluation was performed at a diagnostic 3T MRI scanner (Prisma^fit, Siemens Healthineers, Germany) using a multi spin echo sequence.
Results
It was shown that the design and production of the shape is important (Fig.2). The most homogeneous dose profiles were achieved when neither supporting material, cleaning lye nor with cleaning lye contaminated cleaning accessories get in contact with areas that will later contain PG(Fig.: 2). Further, for the ultrasonic bath, a "cup in cup" variant was chosen in order to exclude contamination with other materials (Fig. 3). During this procedure, the temperature of the bath should not exceed 50°C. When evaluating the irradiated containers, a better polymerisation of the PG was seen with the treatment of isopropanol. Overall, the Objet 500 Connex3^TM showed the strongest minimization of wall effects. When manufacturing a large number of containers, it is advantageous if each container is composed of as few individual parts as possible.
Conclusion
With the optimised workflow described above, containers can be reproducibly produced in large quantity. As wall effects were significantly reduced with the presented method, also small PG containers in anthropomorphic shapes have the potential to be used in E2ET of different treatment scenarios. All findings can be transferred to variable shapes that meet the presented requirements. These findings can be transferred to variable shapes that meet the necessary requirements. Thus, they can also be integrated into anthropomorphic phantoms.
[1] A. Elter et al 2019 Phys. Med. Biol. 64 04NT02