Initial clinical experience with ITV-free Lung SABR using Synchrony tracking on Radixact Tomotherapy
PO-2001
Abstract
Initial clinical experience with ITV-free Lung SABR using Synchrony tracking on Radixact Tomotherapy
Authors: Fabio Menegatti1, Marzia Cerrato1, Cristiano Grossi1, Elena Gallio2, Ilaria Bonavero1, Chiara Casale1, Paolo Brossa3, Serena Badellino1, Francesca Romana Giglioli2, Ramona Parise1, Mario Levis1, Umberto Ricardi1
1University of Turin, Department of Oncology, Turin, Italy; 2A.O.U. Città della Salute e della Scienza, Medical Physics Unit, Turin, Italy; 3A.O.U. Città della Salute e della Scienza, RTT Member, Turin, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
In 2020 Synchrony® was installed on Radixact® tomotherapy at our department. The aim of this study is to report our clinical experience with the use of lung SABR treatments delivered with tomotherapy and Synchrony® respiratory tracking system.
Material and Methods
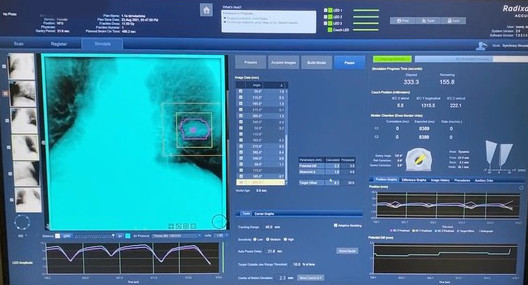
Strict selection criteria included tumor and patients’ characteristics (Table 1). Patients not eligible for surgery with early-stage NSCLC or oligometastatic lung tumors, PS ECOG 0-1, with a single nodule, in peripheral location were included. During the simulation phase, a 4D-CT scan was performed (10 respiratory phases). GTV was contoured on maximum expiration phase scan. PTV was obtained by adding an isotropic expansion margin of 3 mm to the GTV. Tracking target volume was also contoured. A LINAC-based plan was carried out for back-up. In this case an Internal Target Volume (ITV) on the Average scan was acquired from the GTV contoured on the 0% phase scan. Before treatment, patients had to undergo a simulation session (during which the treatment beam is disabled) to verify the feasibility of the respiratory model and patient’s compliance and to test the 2D radiographs consistency (Figure 1). In the event of an unsuccessful simulation phase, patients would be treated according to a LINAC-ITV-based plan. Respiratory toxicity was evaluated with the CTCAE 5.0 grading.
Table 1. Inclusion criteria based on tumor and patients related factors for Radixact® Synchrony® Lung SABR treatments.
Figure 1. Target visualization on Synchrony® respiratory tracking system.
Results
From July 2020 to July 2022, 18 patients were enrolled. Of these, 12 were affected by ES-NSCLC and 6 had lung metastases. The median age was 75 years old. All patients completed the simulation phase successfully and were treated with the Synchrony® plan conforming to a “risk adapted” fractionation schedule (3-5 fractions). The median beam-on time for each fraction was 9.3 minutes (IQ range: 8.6-10.5). The lesion’s maximum transverse diameter of the lesion was 5 cm. Compared with LINAC based plans, a median GTV/PTV ratio reduction of 20.8% was calculated. With a median follow up of 15 months, approximately 67% of patients developed radiological toxicity, mostly asymptomatic or with mild symptoms. Only 3 patients displayed acute G2 toxicity: asthenia, cough, and dyspnoea. No severe pneumonitis of grade 3 or higher was detected.
Conclusion
Our preliminary analysis show the safety and feasibility of Synchrony® tumor-tracking system on a heterogeneous cohort of selected patients with lung lesions. This advanced technical solution allows a reduction of the PTV volume compared to a LINAC based technique without tumor tracking, with an overall treatment time limited to less than 10 minutes and no increased toxicity or discomfort even in more fragile patients.