Toxicity and clinical outcomes to abdominal radiotherapy using 5 fractions for non-surgical patients
PO-1584
Abstract
Toxicity and clinical outcomes to abdominal radiotherapy using 5 fractions for non-surgical patients
Authors: RIE ASSO1, Fabio Cury1, Carolyn Freeman1, Neil Kopek1
1McGill University Health Centre, Division of Radiation Oncology, Montreal, Canada
Show Affiliations
Hide Affiliations
Purpose or Objective
Surgery is the standard treatment for several primary abdominal tumors, including pancreatic and retroperitoneal sarcomas. Abdominal radiotherapy is often considered, either consolidative or as a non-surgical alternative when resection is not possible. Stereotactic hypofractionated radiation therapy (SFRT) offers logistical advantages in terms of both patient convenience but also ease of integration within a multimodality treatment strategy that seeks to minimize delays and interruptions in systemic therapy. In addition, there is a potential radiobiological advantage with respect to overcoming relative radioresistance. Applying SFRT to abdominal tumors, however, could be limited by the tolerance of normal surrounding organs. To analyze toxicity and clinical outcomes in patients treated with five fraction SFRT for non-surgical pancreatic tumours and retroperitoneal sarcomas.
Material and Methods
This is a single institution retrospective review of pancreatic and sarcoma patients treated between 2011 and 2022 with five fractions SFRT to doses between 25 to 30Gy. Cases treated with exclusively palliative intent were excluded, as cases where the SFRT was part of a pre-operative strategy. Patients who were previously resected and/or received chemotherapy were included. Descriptive statistics were used to analyze demographic and disease characteristics. NCI Common Terminology Criteria for Adverse Events version 5.0 were used for toxicity grading. Kaplan-Meier method estimated the clinical outcomes.
Results
49 cases, nine with sarcoma and 40 with pancreas cancer confirmed by pathology, were reviewed. The mean age was 68 years [range: 32-93]. The most common prescription dose was 30Gy (N=25) in five fractions typically delivered on non-consecutive days. The majority of cases received previous chemotherapy (79%), and more than one-quarter (28.5%) had previous surgery for the primary disease. The mean of the tumors was 4.7cm and 43 had a single lesion. During planning, a 4DCT was done to assess for respiratory motion and generate an ITV. Among the 49 patients, 75.5% presented acute side effects. The most common acute side effect was fatigue (42.8%), followed by nausea (24.5%), and abdominal pain (22.4%), all low grade with one case of grade 3 acute abdominal pain. Chronic side effects were observed in 15 patients, abdominal pain being the most frequent. Bowel perforation (N=2) and cholangitis (N=3) were observed in the context of disease progression. At the time of analysis, 81.6% (N=40) of patients had died. Seven patients had no follow-up. Local control (figure 1)/ metastases free survival and OS (figure 3) at 6 months and 1 year: 62.8%/36.4%/61.4% and 42.5%/19.4%/38.3%, respectively. The median overall survival was 9 months.
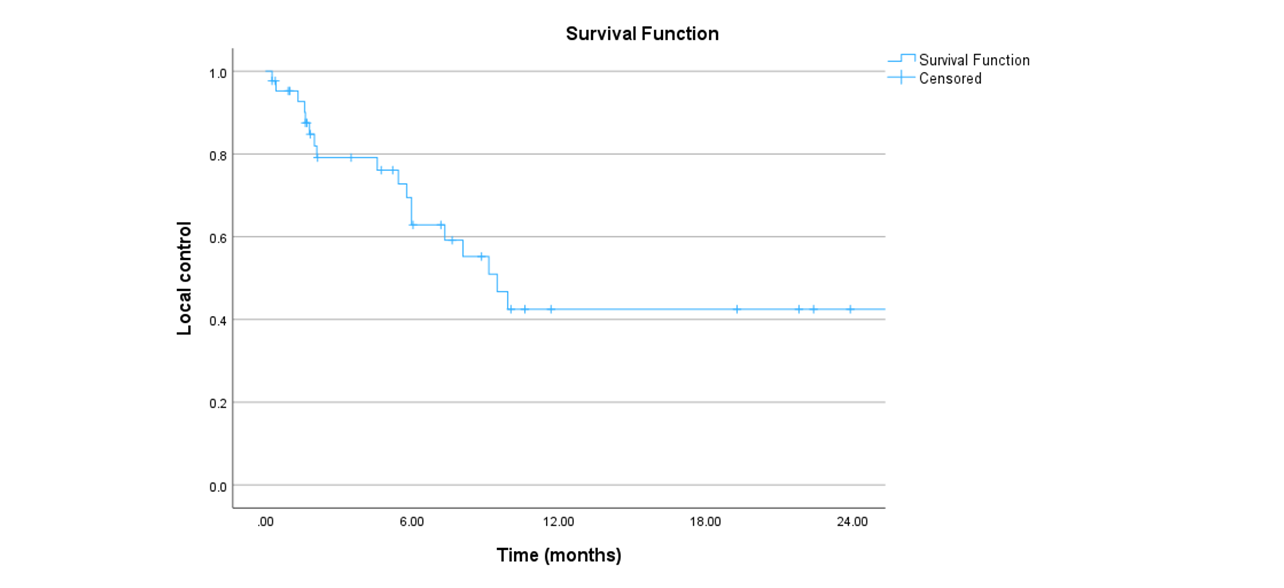
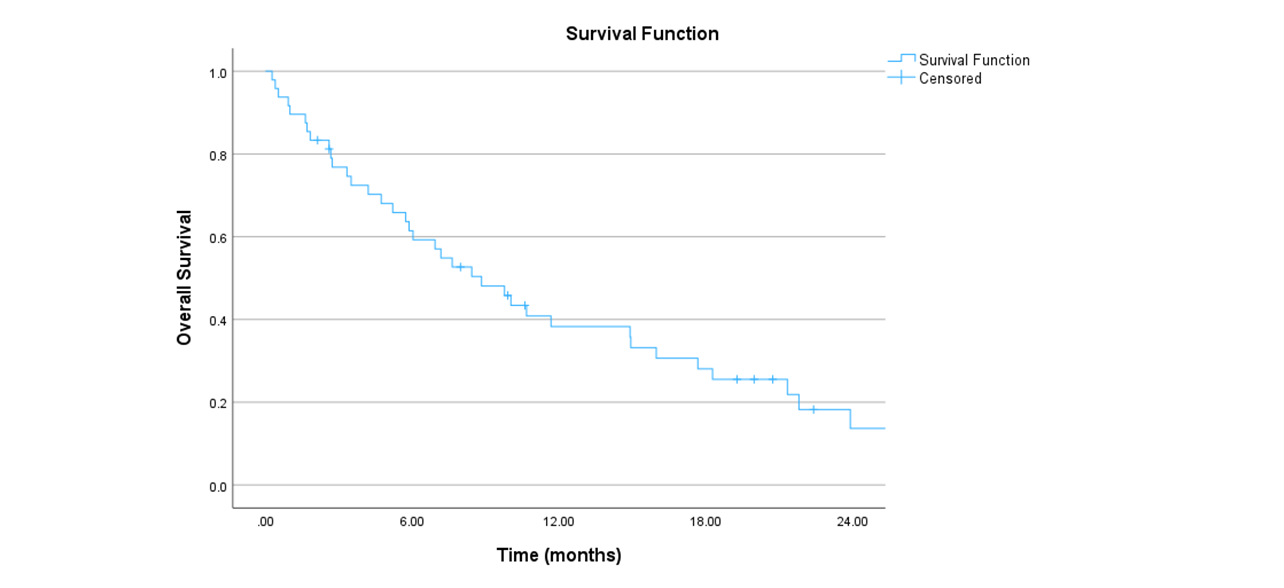
Conclusion
SFRT in five fractions for abdominal tumors appears to be well tolerated with encouraging rates of local control in this cohort of non-surgical candidates.