Degraded 4 MeV electron beam in treating basal cell carcinoma: Safety study
PO-1554
Abstract
Degraded 4 MeV electron beam in treating basal cell carcinoma: Safety study
Authors: Assi Valve1,5, Sari Koskenmies2, Anu Anttonen1, Heidi Nurmi3, Micaela Hernberg4, Mikko Tenhunen1
1Helsinki University Hospital, Cancer Center, Department of Radiation Oncology, Helsinki, Finland; 2Helsinki University Hospital, Department of Dermatology and Allergology, Helsinki, Finland; 3Turku University Hospital, Department of Oncology, Turku, Finland; 4Helsinki University Hospital, Cancer Center, Department of Oncology, Helsinki, Finland; 5University of Helsinki, Department of Physics, Matrena, Helsinki, Finland
Show Affiliations
Hide Affiliations
Purpose or Objective
Basal cell carcinoma (BCC) is developed within the epidermis, and its occurrence increases with age. Typical risk factors are cumulative sun exposure, previous history of BCC, long-term immunosuppression and underlying genetic predisposition. Possible treatment options are determined by tumor’s location and histopathological subtype.
Surgery with complete margin analysis is the golden standard, but many low-risk tumors can be managed with noninvasive options. Radiotherapy can be considered as the primary treatment for inoperable BCC patients [1].
In our trial we used degraded 4 MeV electron beam and 6 x 6 electron applicator from Elekta Versa HD (Elekta AB, Stockholm, Sweden) linear accelerator. The treatment was given as fixed source to skin distance (SSD) 100 cm. The modification of the electron beam has been described in previously published article [2]. Our aim was to perform a safety study, and evaluate, if degraded beam is suitable for treating small superficial BCCs on the body and extremity.
Material and Methods
The trial was conducted during 2019-2022 at Helsinki University Hospital. A patient was accepted to participate, if she had local clinically superficial BCC, thinner than 5 mm and a diameter smaller than 5 cm, located on the body or extremity. An optimal collimator which covered the whole lesion with minimum of 3 mm clinical margin was chosen by the dermatologist.
Patients treated in this trial were divided in two hypofractionation patterns receiving either 5 x 7 Gy, 2-3 times per week, or 2 x 12 Gy, once per week. Biologically equivalent dose with α/β=3 (BED2 Gy) for acute reactions was ~70 Gy for both fractionation patterns, and α/β=10 for tumor was ~50 Gy for longer, and ~44 Gy for shorter pattern. Patients had follow-up checkups after one and six months of last treatment fraction.
Results
The trial included 12 patients (nine men and three women) with 19 lesions. The mean age of the patients was 73.3 years. The most frequent location of the BCC was body, followed by upper arm and legs. The clinical size of the lesions varied from 1 cm to 3 cm.
One month after the treatment the treated lesions were slightly purple and dry, but with no pain or ulcering. The selected treatment pattern or lesion location did not make any difference. After six months the treatment, the targeted lesion was most often slightly hypopigmented. In some cases, hyperpigmentation was seen. The result after one and six months can be seen in Fig 2. All patients found radiotherapy easy with no sense of pain or other adverse events.
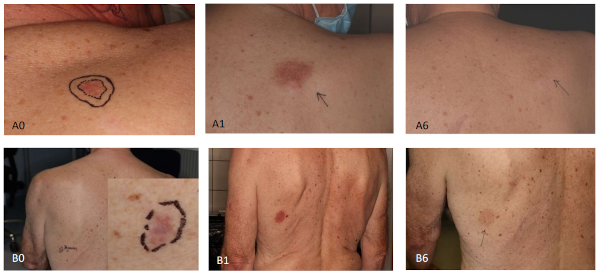
Fig2 The 2 x 12 Gy (A) and 5 x 7 Gy (B) at one (A1/B1) and six (A6/B6) months. A0/B0 is the baseline.
Conclusion
In conclusion, this study showed that both fractionation patterns seemed to give similar outcomes during follow-up time. The patient satisfaction towards the treatment was positive and no adverse events occurred. The method is safe in treating the patients superficial BCC lesions. A response study is going to start with superficial BCC lesions this year.
