Rapid increase in ctDNA at commencement of curative-intent radiotherapy for NSCLC
Michael MacManus,
Australia
MO-0873
Abstract
Rapid increase in ctDNA at commencement of curative-intent radiotherapy for NSCLC
Authors: Michael MacManus1, Benjamin Blyth1, Olga Martin2, Nikki Plumridge1, Mark Shaw1, Fiona Hegi-Johnson1, Shankar Siva1, Owen Banks3, Laura Kirby3, David Ball1, Stephen Wong4
1Peter MacCallum Cancer Centre, Radiation Oncology, Melbourne, Australia; 2University of Bern, Topographic and clinical Anatomy, Bern, Switzerland; 3Peter MacCallum Cancer Centre, Translational Research, Melbourne, Australia; 4Peter MacCallum Cancer Centre, Cancer Genomics Translational Research Centre, Melbourne, Australia
Show Affiliations
Hide Affiliations
Purpose or Objective
A prospective, observational clinical trial was initiated to sequentially monitor a range of circulating biomarkers, including circulating tumour DNA (ctDNA) in patients with non-small cell lung cancer (NSCLC), treated with curative intent radiotherapy or chemoradiotherapy. It was hypothesised that a rapid increase in ctDNA may be observed after the first fraction of radiotherapy as a result of radiotherapy-induced tumour destruction. Preliminary ctDNA results are presented here.
Material and Methods
Patients receiving curative intent for radiotherapy, with or without concurrent chemotherapy, for any stage of lung cancer were recruited. Blood was collected at predefined intervals before, during (including 24 hours after the first fraction of radiotherapy or chemoradiation) and after radiotherapy/chemoradiotherapy. Patients had baseline and post treatment FDG-PET scans and were followed up for clinical outcomes, including progression-free survival. Tumour specific mutations were identified through routine molecular diagnostic testing, with mutation specific droplet digital PCR assays used to track ctDNA levels throughout treatment.
Results
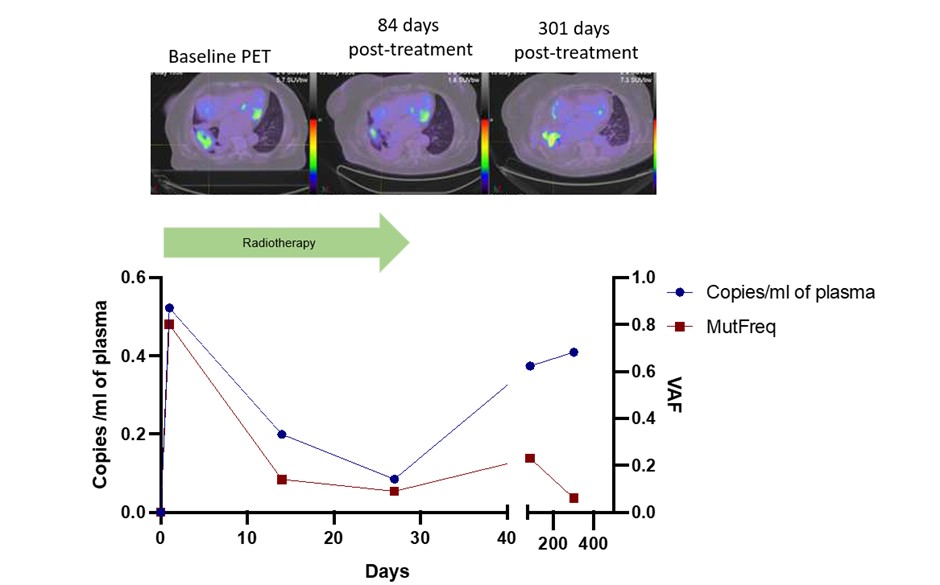
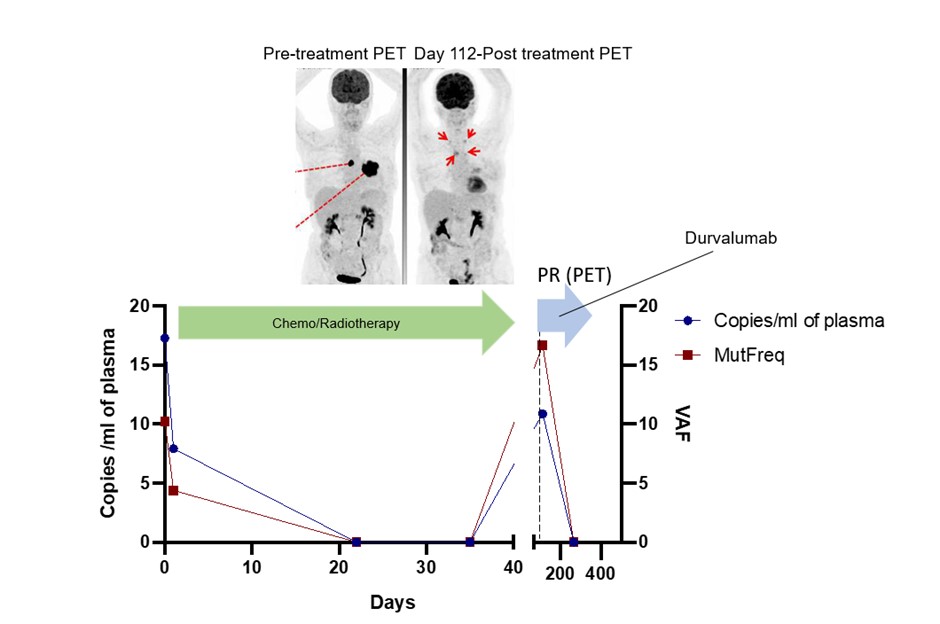
Seventy four of a planned 100 patients have so far been recruited to this ongoing study. Sequential ctDNA results are currently available for 7 patients, with follow-up of 81 to 552 days (median 266 days). Known tumour-based mutations including in EGFR, KRAS and TP53 that were assayed. Treatments delivered were fractionated radiotherapy (n=6) or SABR (n=1). An increase in ctDNA was observed after the first fraction of radiotherapy in 4 of 5 patients treated with fractionated radiotherapy with a complete set of results, including in 2 cases where ctDNA was initially undetectable prior to treatment (representative case of ctDNA “spike” shown in upper figure). The patient treated with SABR had small volume stage I disease and ctDNA was undetectable before or soon after radiotherapy but became detectable at the time of distant metastasis 552 days post treatment. In other cases, the ctDNA levels mirrored the clinical course. One patient who had a partial response to chemoradiotherapy had detectable ctDNA at the end of treatment but attained molecular remission when adjuvant durvalumab was initiated (see lower figure).
Conclusion
Serial ctDNA analysis may provide clinical useful information. A “spike” in ctDNA was detected after the first fraction in 4 of 5 patients treated with fractionated radiotherapy. This phenomenon, which may represent rapid initial tumour cell destruction, could have clinical value as a surrogate for early treatment response and as a means of enriching plasma for ctDNA for mutational profiling.