Sarcopenia - a prognostic biomarker for definitive chemoradiotherapy in oesophageal cancer patients
MO-0875
Abstract
Sarcopenia - a prognostic biomarker for definitive chemoradiotherapy in oesophageal cancer patients
Authors: Donal McSweeney1, Sophie Raby2, Jamie Weaver2, Ganesh Radhakrishna2, Andrew Green3, Paul A. Bromiley4, Marcel van Herk1, Alan McWilliam1
1University of Manchester, Division of Cancer Sciences, Manchester, United Kingdom; 2The Christie Hospital NHS Foundation Trust, Medical oncology, Manchester, United Kingdom; 3EMBL European Bioinformatics Institute, Protein Sequence Resources, Cambridge, United Kingdom; 4University of Manchester, Division of Informatics, Imaging and Data Sciences, Manchester, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
Sarcopenia is an emerging biomarker of patient frailty that is associated with increased toxicity and decreased survival in a number of cancer types. Definitive chemoradiotherapy (dCRT) is the standard of care for patients with unresectable oesophageal cancer (OC). But research on the role of sarcopenia in this population is lacking. Sarcopenia is usually assessed by segmenting skeletal muscle (SM) at the L3 vertebral level. For OC patients treated with radiotherapy (RT), planning scans do not always include L3. The ability to assess SM at other vertebral levels would also enable longitudinal studies of body composition.
We therefore investigate the prognostic value of SM area, assessed at T12, in non-metastatic OC patients treated with dCRT.
Material and Methods
RT planning CTs were collected for 140 patients treated with dCRT (50 Gy in 25 fractions; carbo-taxol or cis-cap) between 01/01/2017 and 01/10/2020. T12 was manually identified and SM was automatically segmented by an in-house deep-learning model. Failed segmentations were manually corrected. SM index (SMI) was defined as single slice muscle area after thresholding between -29 HU and 150 HU (to account for intra-muscular fat) normalised by patient height squared.
The prognostic value of SMI was investigated in Kaplan-Meier curves and multivariable Cox models accounting for age, sex, performance status (PS), feeding tube insertion pre-RT, O’Rourke dysphagia score, T & N stage, histology, specific tumour site and pre-RT BMI. The interaction between BMI and SMI was also investigated. The primary endpoint was overall survival (OS).
Results
Automatic segmentation was successful for 96% of cases. Kaplan-Meier curves split on sex-specific median SMI showed a significant difference in OS (p=0.025; Fig1(a)). Sarcopenic patients had a median survival time of 534 days compared to 993 days for non-sarcopenic patients.
In Cox models, SMI was found to be a prognostic factor (p=0.04; Fig2) alongside pre-RT feeding tube insertion, histology and N stage, where increased SMI was protective. The interaction between BMI and SMI was also a significant factor (p=0.04), which suggests that the effect of SMI on OS varies with BMI. This is shown in Fig1(b) where the effect of SMI on OS is different for different BMI sub-groups (underweight & normal vs. overweight & obese). The adverse effect of low SMI was strongest in low BMI patients. Whereas, SMI was not an independent prognostic factor in overweight/obese patients.
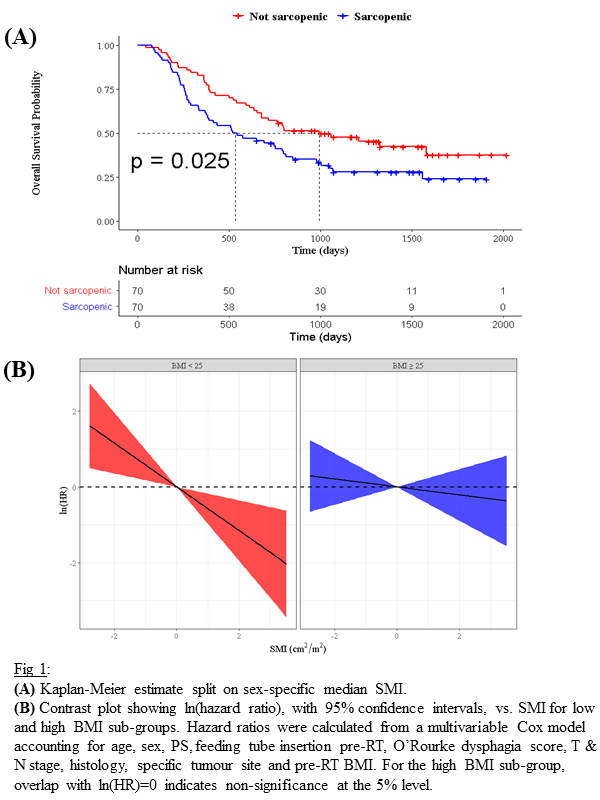

Conclusion
SMI evaluated at T12 is an independent prognostic factor for OS in non-metastatic OC patients treated with dCRT. SMI provides additional prognostic value over BMI and PS. Patients with low BMI and low SMI experienced the worst outcomes. At higher BMI the prognostic nature of low SMI was removed, emphasising the importance of close nutritional support in this population. Although external validation is required, this work suggests that a full understanding of body composition, over BMI alone, is needed for prognostic models in OC.