The Impact of 2019 ESTRO-ACROP target volume delineation guideline on breast-related complications
JUNG BIN PARK,
Korea Republic of
MO-0141
Abstract
The Impact of 2019 ESTRO-ACROP target volume delineation guideline on breast-related complications
Authors: JUNG BIN PARK1, Bum-Sup Jang1, Ji Hyun Chang1, Jin Ho Kim1, Ki Yong Hong2, Ung Sik Jin2, Hak Chang2, Yujin Myung3, Jae Hoon Jeong3, Chan Yeong Heo3, In Ah Kim4, Kyung Hwan Shin1
1Seoul National University Hospital, Radiation Oncology, Seoul, Korea Republic of; 2Seoul National University Hospital, Plastic and Reconstructive Surgery, Seoul, Korea Republic of; 3Seoul National University Bundang Hospital, Plastic and Reconstructive Surgery, Seongnam, Korea Republic of; 4Seoul National University Bundang Hospital, Radiation Oncology, Seongnam, Korea Republic of
Show Affiliations
Hide Affiliations
Purpose or Objective
The European Society for Radiotherapy and Oncology Advisory Committee in Radiation Oncology Practice (ESTRO-ACROP) recently updated a new target volume delineation guideline for postmastectomy radiotherapy (PMRT) after implant-based reconstruction. This study aimed whether this change has impact on breast-related complications.
Material and Methods
We retrospectively reviewed patients who underwent PMRT after mastectomy with tissue expander or permanent implant insertion from 2016 to 2021. In total, 400 patients were included; 264 received RT by the new ESTRO-ACROP target delineation (ESTRO-T), and 136 received RT by conventional target delineation (CONV-T). The primary endpoint was comparison between the target groups of major breast-related complication, including infection, capsular contracture, deformity and necrosis requiring re-operation or re-hospitalization during follow-up after RT or delayed implant replacement. Complications were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0., and capsular contracture was graded by the Baker Classification.
Results
The median follow-up was 29.5 months (range, 0.3-76.8). The 1-, 2-, and 3-year incidence rates of major breast-related complication were 5.4%, 9.4%, and 12.0% in the ESTRO-T group, and 8.2%, 13.9%, and 14.8% in the CONV-T groups; it did not show a difference between the groups (P = 0.52, Figure 1.(A)). In multivariate analyses, target delineation is not significantly associated with the major complications (hazard ratio [HR] = 0.93; P = 0.83). There was no significant difference between the ESTRO-T and CONV-T groups in the incidence of any breast-related complications (3-year cumulative incidence, 36.0% vs. 30.1%, respectively; P = 0.29, Figure 1.(B)). Symptomatic RT-induced pneumonitis rates were 2.7% in the ESTRO-T group (7 patients) and 2.2% in the CONV-T group (3 patients). Only one local recurrence event occurred in the ESTRO-T group, which was within the ESTRO-target volume.
Figure 1.
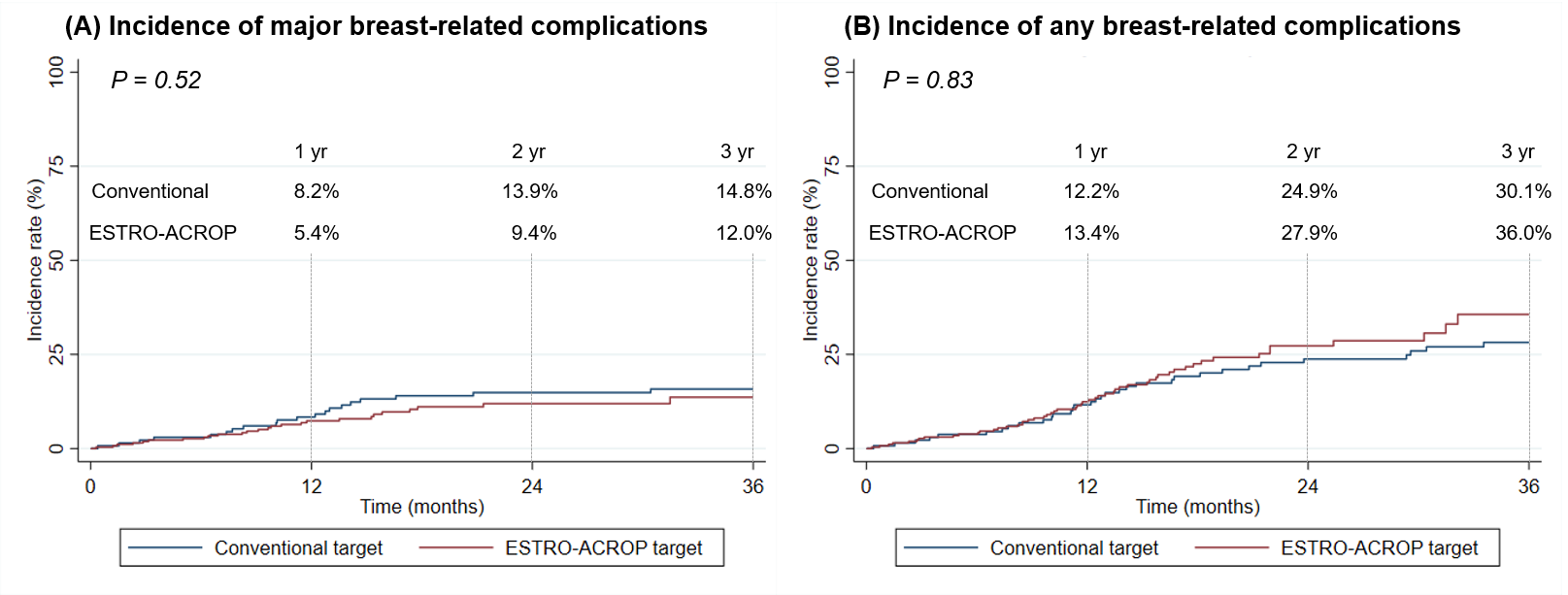
Conclusion
Target volume delineation according to the new ESTRO-ACROP guideline did not reduce the risk of major or any breast-related complications. As the dosimetric benefits of heart and lung have been reported, further analyses with long-term follow-up are necessary to evaluate whether it could be connected to better clinical outcomes.