A randomized trial of adjuvant hyperthermia to RT of locally advanced breast cancer, ESHO 1-85.
MO-0135
Abstract
A randomized trial of adjuvant hyperthermia to RT of locally advanced breast cancer, ESHO 1-85.
Authors: Jens Overgaard1, Maarten CCM Hulshof2, Olav Dahl3, Giorgio Arcangeli4
1Aarhus University Hospital, Department of Experimental Clinical Oncology, Aarhus , Denmark; 2Amsterdam University Medical Centers, University of Amsterdam, Department of Radiotherapy , Amsterdam, The Netherlands; 3Haukeland University Hospital , Department of Oncology, Bergen, Norway; 4Regina Elena National Cancer Institute, ., Rome, Italy
Show Affiliations
Hide Affiliations
Purpose or Objective
The ESHO protocol 1-85 is a international multicenter randomized trial initiated by the European Society for Hyperthermic Oncology with the aim to investigate the value of hyperthermia (HT) as an adjuvant to radiotherapy (RT) in treatment of locally advanced breast carcinoma. The trial is one of the largest studies of hyperthermia in radiotherapy but has not been previously published.
Material and Methods
Between February 1987 and November 1993, 155 tumors in 151 patients (4 patients had simultaneous bilateral tumors). Tumors were stratified according to institution and (T2-3/T4) and randomly assigned to receive radiotherapy alone (2 Gy/fx, 5 fx/wk) to a total dose of 65-70 Gy, incl. boost, or the same radiotherapy followed once weekly by hyperthermia (aimed for 43 °C for 60 min). Radiation was given with high voltage photons or electrons. The primary endpoint was persistent complete response in the treated area.
Results
A total of 147 tumors in 143 patients were evaluable, with a median observation time of 21 (range 1-134) months. Seventy tumors were randomized to RT alone and 77 to RT+HT. Size was T4 in 92, and T2-3 in 55 tumors, respectively.
The compliance to RT was good with all but 12 pts fulfilling the planned RT treatment. The tolerance to HT fair, but associated with moderate to severe pain and discomfort in 15% of the treatments. In 83% of the heated patients a least one heat treatment achieved the target temperature, but the temperature variations was large. Addition of heat did not significantly increase the acute nor late radiation reactions.
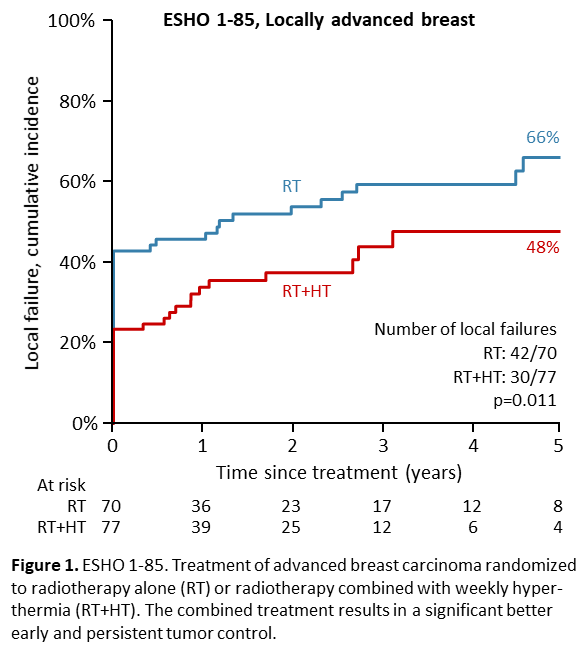
Overall, the 5-year actuarial local failure rate 57%. Univariate analysis showed a significant influence of hyperthermia (RT alone 66% versus RT+HT 48%, p = 0.011, see figure 1) and T- (T4 72% versus T 2-3 35%, p = 0.003). A Cox multivariate analysis showed the same factors to be the only significant prognostic parameters: hyperthermia (HR: 0.58 [0.36-0.94], p = 0.02) and tumor (odds ratio: 0.49 [0.29-0.83], p = 0.006). Consequentially, more patients given RT+HT (38%) survived without disease (DFS), than after RT alone (20%), p = 0.019)
Conclusion
A randomized multicenter trial investigating the addition of a weekly hyperthermia treatment to radiotherapy of patients with locally advanced breast cancer significantly enhanced the 5-year tumor control and yielded more patients surviving free from cancer. The results of this Phase III prospective multicenter randomized trial substantiate the potential clinical benefit of hyperthermic oncology.