Compliance to the PATHOS swallowing OAR atlas and impact on predicted dysphagia for trial patients
Emma Higgins,
United Kingdom
MO-0712
Abstract
Compliance to the PATHOS swallowing OAR atlas and impact on predicted dysphagia for trial patients
Authors: Emma Higgins1, Nachi Palaniappan1, Richard Webster1, Zohal Nabi2, Rikki Lad3, Kate Elliott3, Elizabeth Miles3, Joanna Canham4, Lisette Nixon5, Chris Hurt4, Christie Heiberg5, Matt Beasley6, Terry Jones7, Mererid Evans1
1Velindre Cancer Centre, Clinical Oncology, Cardiff, United Kingdom; 2Mount Vernon Cancer Centre, RTTQA Group , Northwood, United Kingdom; 3Mount Vernon Cancer Centre, RTTQA Group, Northwood, United Kingdom; 4Cardiff University, Centre for Trials Research , Cardiff, United Kingdom; 5Cardiff University, Centre for Trials Research, Cardiff, United Kingdom; 6Bristol Haematology and Oncology Centre, Clinical Oncology, Cardiff, United Kingdom; 7University of Liverpool, Molecular and Clinical Cancer Medicine, Liverpool, United Kingdom
Show Affiliations
Hide Affiliations
Purpose or Objective
PATHOS is a phase III RCT investigating whether de-intensified adjuvant treatment after transoral surgery for patients with HPV related Oropharyngeal SCC, will result in improved swallowing function, whilst maintaining excellent clinical outcomes.
As part of the Radiotherapy Trial Quality Assurance (RTTQA) programme, investigators were invited to delineate 9 Swallowing OAR (SWOARs) in a swallowing sub-study. Inaccurate SWOAR delineation may affect plan optimisation, lead to inconsistencies in dose delivery to normal tissues and potentially impact NTCP.
This study aims to evaluate compliance with the PATHOS contouring atlas and determine if variation in SWOAR delineation leads to any difference in physician-scored dysphagia at 6 months (NTCPD6).
Material and Methods
The dataset consisted of 85 patients recruited between Dec 2015 and Feb 2020 that had SWOAR contours submitted. A gold standard (GS) set of outlines was contoured on all cases by a single investigator (EH) and checked by two senior clinicians.
A qualitative retrospective analysis was performed whereby the submitted SWOAR contours (SC) were categorised using the same principles adopted by the RTTQA team: “conforms to protocol”, “acceptable variation where study outcome is unlikely to be affected” and “unacceptable variation where study outcome could be affected.”
The predicted NTCPD6 for each case of the GS and SC was calculated by applying the predicted model of Christianen et al [NTCPD6 = 1/(1 + e)-S, where S = −6.09 + (mean dose PCM_Sup × 0.057) + (mean dose Larynx_SG × 0.037)]. The differences between the NTCPD6 for GS and SC were then compared.
Results
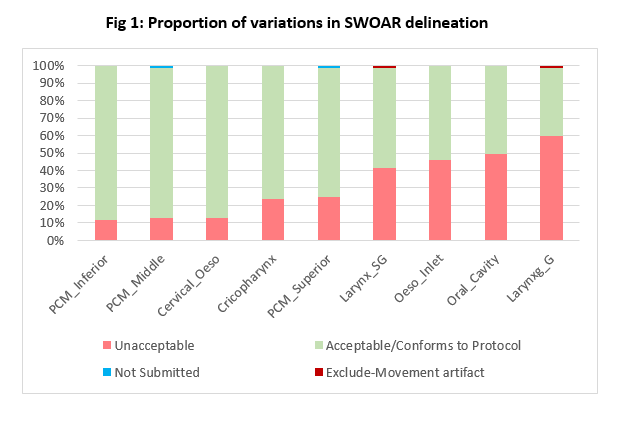
9 SWOARS were reviewed on 85 cases-765 contours in total. Variation in delineation was observed in all submitted SWOARS (Fig 1), with the Larynx_G having the most unacceptable variations (60%), mainly due to arytenoids being excluded or air not edited out. Large variations were also seen in the Oral_Cavity (49%), Oeso_Inlet (46%) and Larynx_SG (41%) mostly due to incorrect lateral border at the mandible, incorrect inferior border of the cricoid, and air not edited out respectively. Overall, 32% contours had unacceptable variations.
The predicted NTCPD6 differences between GS and SC ranged from 3.4 to -6.2%. Larger differences were seen in those cases with unacceptable variations in Larynx_SG or PCM_Superior (Fig 2).

Conclusion
Protocol compliance to the PATHOS contouring atlas showed unacceptable variations in SWOAR delineation in 32% cases. These variations are of no clinical significance within the trial as treatment plans are not optimised to SWOARs.
Of note, larger differences were present in the predicted NTCPD6 between GS and SC when the contours of the relevant SWOARS did not conform to protocol. Accurate SWOAR delineation is therefore an essential step in NTCP calculation and an important consideration when using NTCP models e.g.in plan optimisation or patient selection for treatment techniques.
(PATHOS trial no: A25317)