ESTRO 2023 Young Report on Interdiscplinary Track
ESTRO and the European Organisation for Research and Treatment of Cancer (EORTC): ‘Next generation clinical trial design: a multidisciplinary effort’
It was a delight to participate in this year’s ESTRO congress once again. One of the hidden pleasures is attending the joint sessions to see what is happening or what is in store for us from all the different groups that interact with each other. This year I believe the ESTRO-EORTC joint session on the next generation of clinical trials was of particular interest for the future. Key issues that face radiation oncology trials were highlighted by Corinne Faivre-Finn. These included the provision of funding mainly by governments or charities and the scarcity of industry-driven studies; and a lack of equipoise or combination of therapeutic modalities with other specialties.
To address these issues, several ideas were put on the table such as increased consideration of patient-centred questions, the use of more pragmatic designs, a decluttering of the case-report form or even simplification of the patient consent process. Such approaches are already being implemented in projects such as the Concorde phase I platform. Likewise, with randomised controlled trials enrolling only a small portion of patients and, in the majority of cases, not reflecting the true patient populations that we see in our daily practices, it was proposed that to consider some research questions, we would have more success looking at real-world data in the form of cohort studies such as OligoCare.
We were presented with not just difficulties but success stories, such as the trial of systemic therapy in advancing or metastatic prostate cancer and the evaluation of drug efficacy (the STAMPEDE trial) by Nicholas James and the design benefits that made it successful. These benefits were: the use of multiple arms that employed simple and broad inclusion criteria and simplified endpoints; and that it was possible to answer multiple questions that materialised over an extended period through the use of the already existing platform.
One of the pillars of modern radiotherapy is the use of radiotherapy quality assurance (RTQA) to ensure that clinical trials test high-quality radiotherapy treatments. A very thought-provoking talk about the cost of RTQA as an intervention in clinical trials touched on this subject. It was given by Enrico Clementel. A simulation was presented to us regarding expenses of clinical trials, which included multiple levels of RTQA procedures and their impact on trial duration.
Lastly, patient involvement in the designs of clinical trials was addressed by Helen Bulbeck. She reminded us that although we may focus on a multitude of endpoints and improving technology, in the end the things that matter the most to patients are quality of life and symptom relief.

Daniel Portik
Radiation oncologist
EORTC headquarters
Brussels, Belgium
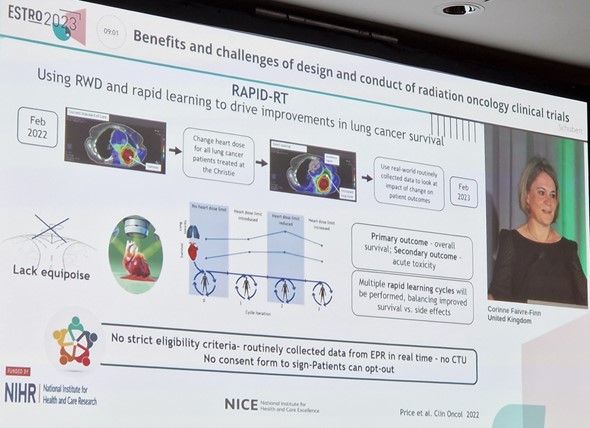
Presentation from Corinne Faivre-Finn
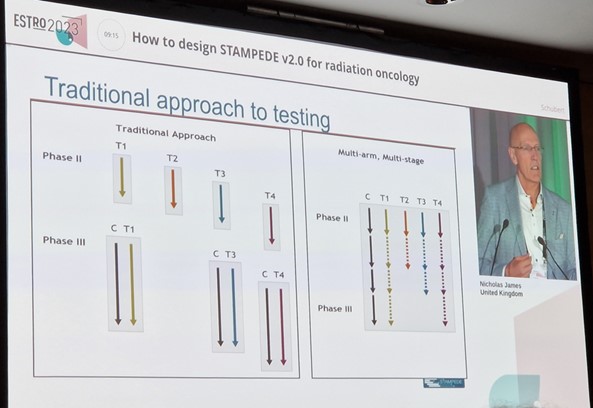
Presentation from Nicholas James