A visionnary in radiotherapy and medical physics
By Marcel van Herk, Chair in Radiotherapy Physics, University of Manchester, UK

Fig. 1: Ben Mijnheer 18 October 1941 – 29 May 2024
Since 2005, the Netherlands Cancer Institute (NKI) has successfully used portal dosimetry to record and verify all IMRT patients, providing both evidence that patients were treated as planned, and to catch various cases of dose delivery discrepancies [1, 2]. This triumph was the vision of Ben Mijnheer (Fig. 1), when together with Harm Meertens, they described portal dosimetry in a Dutch Cancer Foundation-funded project back in 1983. Today, portal dosimetry is a commercial product, initially released by Elekta in 2014 and now by PTW, helping the world to efficiently and accurately verify radiation dose delivery.
Bernard Jochem Mijnheer was born on the 18th of October, 1941 and passed away after a long illness on the 29th of May, 2024. He continued working until very recently, as is evidenced by his contribution to AAPM task group report 307, published in 2023 [3], and being chief editor of the book, "Clinical 3D Dosimetry in Modern Radiation Therapy ", published in 2018 [4]. The focus of Ben's career was a quality assurance of radiotherapy and the development of new treatment techniques. His legacy includes over 250 publications including 21 book chapters, 25 PhD theses and 1 book. Ben was awarded the ESTRO-Breur award in 1998 (Edinburgh, Fig. 2), the ESTRO van der Schueren award in 2013 (Geneva) and the ESTRO Lifetime Achievement Award in 2016 (Turin).

Fig. 2: Ben Mijnheer receiving the ESTRO-Breur award in 1998 in Edinburgh.
After high school, Ben began his training in Nuclear Chemistry. He completed his PhD at the University of Amsterdam (1971) on neutron measurements [5], and followed this with a postdoc at the Central Bureau of Nuclear Measurements, Euratom in Belgium. Ben was then hired by NKI in Amsterdam in 1973, as a Research Fellow to work on neutron therapy (Fig. 3). Expecting to perform measurements and calculate dosages, he was surprised to find that he was actually required to treat the first patients himself, since no radiographers were available [6]. He dealt with this situation in his typical positive style, demonstrating he was definitely cut out to be a Medical Physicist. One of his earliest papers related to depth-dose characteristics of neutron sources [7]. Some of his published work from this period included a study on the effect of the finite size of ion chambers for dosimetry of neutron beams [8], in vivo dosimetry [9], treatment planning [10], and international dosimetry comparisons [11]. He also co-authored the first European protocol for neutron dosimetry in 1981 [12].

Fig. 3: Neutron radiotherapy in NKI with radiographer Josien de Bois around 1979. Ben Mijnheer was in charge of dosimetry and planning for this machine.
The clinical results of neutron therapy were disappointing, and the project was abandoned in 1981 [13]. Ben was soon appointed Senior Medical Physicist, and this was to be his job for the rest of his life. His knowledge in neutron therapy would, however, later be applied in developments for boron-neutron capture therapy [14].
In keeping with themes from his earlier work in neutron therapy, Ben remained interested and dedicated to the field of dosimetry, treatment planning and international standardisation of dosimetry protocols. For me, a crucial, foundational product from these early years was his recommendation on the accuracy of radiotherapy, which was derived from an extensive review of the available literature on dose-effect relationships [15]. This recommendation would inform later recommendations by the IAEA [17], ICRU and AAPM.
It was an exciting time. NKI's first CT scanner would be installed in 1982, and new planning systems were being developed for neutrons and photons. Rob van der Laarse developed one of the first inverse planning systems in 1976 [17] and algorithms for computerised electron beam planning were being developed [18]. The department collaborated with Nucletron in the development of afterloaders and treatment planning systems for brachytherapy. Many international guests visited our department such as Anders Brahme in 1986, and John Wong, Alan Nahum and J van de Geijn in 1987. The transition from 2D treatment planning and simulator films into CT-based treatment planning happened around this time.
Ben’s insight was that as treatment techniques became more complex, and their preparation and execution more and more split between different disciplines, information may fall between the gaps and the likelihood of errors may increase, both on an individual patient basis, or even for groups of patients. The introduction of in vivo dosimetry for each treatment would close these gaps and help achieve fundamentally safer treatments.
I first came into contact with Ben in 1985, when I was hired as PhD student on the Dutch Cancer Foundation project mentioned above. The goal was to solve the geometric and dosimetric quality assurance issues in radiotherapy once and for all. I started developing an electronic portal imaging device (EPID) during my master's project, under the supervision of Harm Meertens [19, 20]. I worked with Ben for 30 years (Fig. 4), until 2015 when I left the NKI to move to Manchester.

Fig. 4: Ben Mijnheer, Department Chair Harry Bartelink and Marcel van Herk at a party in NKI in 2007
As supervisor Ben was caring, fair and honest, and he would correct any article very quickly and accurately with his propelling pencil. He told me “I do it right away – with a glass of red wine – to avoid it stacking up”. Ben told you how it was, quite directly, and sometimes I would not be happy with his corrections – but he was always right. He was utterly honest without any hidden agenda and often said “Do not worry about sharing your ideas, there is always more to research”. Many of his students now lead clinical departments or research teams.
Ben became head of the research division of the Radiotherapy Department in 1982 and initiated a weekly research committee meeting. The tasks of that meeting, including physicists, engineers and clinicians, involved brainstorming new projects and providing independent reviews of project proposals, articles and abstracts. Under his leadership, our group grew in quantity and quality. At that time 95% of our external grant proposals were granted, as former Chair Harry Bartelink proudly told me recently. Our research was also rapidly translated into clinical practice, first into our own department, and later commercially to reach other clinics worldwide. I think his collaborative way of joining forces between research activity and the clinic, to constantly improve and promote high treatment standards, should be a role model for any radiotherapy department. Ben indicated that a lack of collaboration between clinics and research groups is the most serious threat to innovation [6]. On a more personal level, Ben showed an immense amount of care for his role by making the rounds of the machines every night around 7 pm, to stimulate and support the researchers working there. His collaborative efforts also extended beyond the department, evidenced by the regular dinners he organised for international guests and students.
In 1986, Ben branched out and undertook a sabbatical in Boston, working with Lee Chin to improve photon treatment planning. They studied the incorporation of electron transport [21], performed protocol comparisons [22] and developed planning protocols for total body irradiation [23]. In total, this collaboration produced 8 publications.

(A)
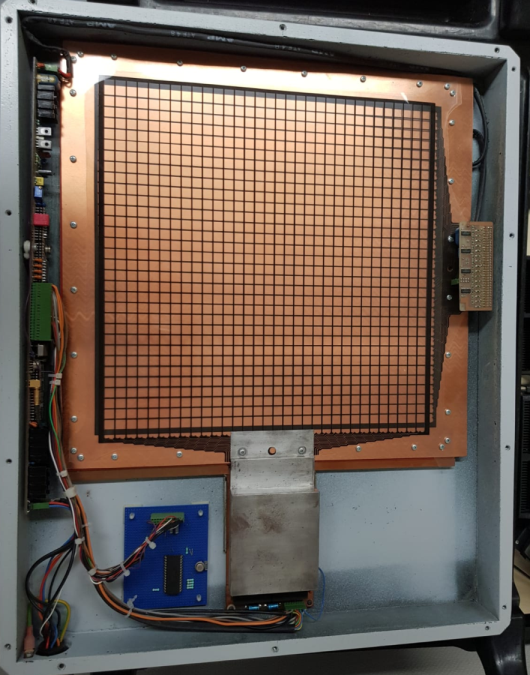
(B)
Fig.5: A) Hand-written letter by Ben Mijnheer as sent to the author in 1986 proposing transmission dosimetry. B) Early prototype of a transmission dosimeter developed by the author according to this idea. Later, standard EPIDs would be used for this purpose. Note that the housing was made of carbon fibre – one of the earliest applications of this material in radiotherapy.
While in Boston, Ben would regularly write me letters to inquire about my progress and to stimulate the development of EPID dosimetry (Fig. 5a). However, it took a few years before we started building prototypes, because the development of the imaging system and image processing software took priority for me. The EPID used for imaging was a scanned ionisation chamber containing isooctane [20]. As a dosimeter, this detector was not very stable because of changing pollution levels in the liquid. To overcome this problem, we built a low-resolution air-filled ion chamber array in 1989 (Fig. 5b). This, however, had a very low resolution due to the lack of shielding between the chambers and would also be unstable due to the accumulation of static electricity on isolating surfaces in the chambers.
For that reason, Ben introduced in vivo dosimetry via the use of entrance and exit diodes [24, 25]. EPID images would be used to describe the location of the diodes and correct for self-attenuation [26]. Later, liquid-filled detectors were more stable, and we reached some success by taking into account the non-linear response of the liquid ion chamber due to recombination [27]. We started with transmission dosimetry [28], and ultimately developed 2D in vivo portal dosimetry for the liquid ion chamber detector, using convolution algorithms [29]. Rob Louwe applied this EPID to in vivo dosimetry for breast radiotherapy [30]. Under our guidance as co-supervisors, student Ronald Boellaard was particularly proficient and would go on to become our first student to reach full professorship in 2012, albeit in nuclear medicine.
EPID dosimetry developments accelerated with the arrival of aSi flat panel imagers in 2001 that had much better characteristics for imaging and dosimetry. Amongst others, Leah McDermott studied detector characteristics and clinical implementation [31, 1], Markus Wendling refined the algorithms for 3D in vivo dosimetry [32], Anton Mans developed the system for VMAT and reported on over 4000 treatments [2], Roel Rozendaal studied the effect of anatomical changes [33], Hanno Spreeuw accelerated 3D dose reconstruction [34], Iban Torres-Xirau implemented EPID dosimetry for the MR-linac [35] and Igor Olaciregui-Ruiz simplified commissioning, advanced the algorithm and automated the workflow [36]. All this research activity and clinical work over many years, was undertaken with Ben’s constant guidance and absolute adherence to best dosimetry practices.
Besides in vivo dosimetry, Ben excelled in the development of new treatment techniques. In this endeavour, he was helped by many physicists, often from abroad. One of them was Dick Fraass of Michigan University, who joined us for a sabbatical at NKI in 1994 and again in 2005 to work with Ben to develop integrated boost plans for prostate [37] and IMRT for sinus cancer [38]. Dick was also instrumental in the introduction of CT-based planning at NKI in 1994, using their in-house system U-MPlan that would be briefly commercial as ScandiPlan. Dick Fraass writes about Ben: “Wendy and I hope that you will let all of Ben's family, colleagues, and friends know how much we appreciated Ben's friendship, his help, his sense of humour, and his boundless enthusiasm for life. Scientific sparring with Ben was an Olympic sport and was always well-meaning and lots of fun - for everyone. His openness and friendliness was unbounded, as was his excitement for the possibilities that some research might bring." Around 2001 NKI transitioned from conformal radiotherapy to IMRT using simple segmented techniques [39]. IMRT optimisation work was done in collaboration with Radiation Oncologists Joos Lebesque for prostate [37] and lung [40], Harry Bartelink for breast [41], and Coen Rasch for head and neck cancer [42].
Ben also stimulated engineers and radiographers to pursue a scientific career, which was unusual at the time. He told Marcel Steggerda, working on brachytherapy, to publish his work in what was to be the first of many papers [43]. Marcel Steggerda completed his PhD in 2010. Between 2003 and 2009 Ben was a lector at InHolland University where radiographers are trained (Fig. 6). One of the radiographer outputs from NKI is a comparison between IMRT and conformal RT for parotid gland tumours [42]. Radiographer Helen from London told me: “Ben stimulated me to submit my first ESTRO abstract”. Now, 25 years later, she is about to become a full Professor in Radiography. In the same period that Ben was lector at InHolland, he also played an advisory role at Maastro in Maastricht, leading to several publications [e.g. 44]. After 2009, he remained a guest researcher at NKI until his death.

Fig.6: Ben Mijnheer at his farewell symposium at InHolland University for radiographers in 2009.
Ben played a major role in the Dutch Clinical Physics Society (Fig. 7) and was the Chairman of the Netherlands Committee on Radiation Dosimetry, NCS, overseeing the publication of many Dutch standards in dosimetry that are also used internationally [45]. The dosimetry protocols he wrote at this time are still in use today [e.g. 46]. Ben also authored and edited leading European radiotherapy guidelines, the so-called ESTRO booklets, two about dose calculation [47, 48], one about quality assurance of treatment planning systems [49] and one about treatment verification [50]. He also was co-founder of the European Institute for Radiotherapy aiming to produce standard guidelines [51].

Fig. 7: Meeting of the Dosimetry Workgroup West Netherlands at NKI in 1985. Ben is sitting middle back.
It was Ben’s idea to improve the representation of clinical physics at ESTRO, and he chaired the first and second ESTRO Biennial Meeting on Physics in Clinical Radiotherapy. He co-chaired until the 7th and last ESTRO Biennial Meeting on Physics in Clinical Radiotherapy in Geneva. He was also a member of the scientific committee for countless other ESTRO meetings. About ESTRO he said: “I enjoy its typical European flavour, working together with people from different cultures.” He had several projects with ESTRO, including Emperion, a joint curriculum development for Medical Physicists in Europe, along with, amongst others, Germaine Heeren from ESTRO (Fig. 8). However, unfortunately still Medical Physicists cannot easily move between European countries, due to persistent differences in education as well as language barriers [6]. He was also part of the AAPM task groups on the effect of Hip Prostheses in radiotherapy [53], EPID dosimetry [3] and Quality Assurance (TG-100) and the BIR working party on uncertainties in external beam radiotherapy [53].

Fig. 8: Group photo of the Emperion project team developing the European medical physics training curriculum in 2006. Far left is his good friend and ESTRO administrator of the first hour Germaine Heeren, and far right Ben Mijnheer.
Ben organised many Radiotherapy Physics courses (Fig. 9), such as the ESTRO Teaching Course in Radiation Physics for Clinical Radiotherapy (Leuven Course) 1985-1997. He was course director for the ESTRO Teaching Course on Conformal Therapy and Other Advanced Treatment Techniques (Amsterdam 1996-1999), which would later become the ESTRO Teaching Course on IMRT and other Conformal Techniques in Practice (course director, Amsterdam 2000-2004). The teachers together published a plan evaluation of the homework case [38]. He would regularly teach worldwide through ESTRO, IAEA and local organisations. Ben was a gifted speaker, and students lauded his kindness. On one of these courses in Poland, he met physicist Mariia Gavrylenko-Mijnheer, who later became his second wife (Fig. 10). After 8 years of dating they married in secret in Phoenix, USA in 2012, but gave a big party a few months later in Amsterdam.

Fig. 9: Ben Mijnheer hands-on teaching dosimetry, 1985.

Fig. 10: Ben Mijnheer and his wife Mariia Gavrylenko-Mijnheer at AAPM 2017 in Denver, Colorado
He is also revered by his secretaries. Patricia Fewer has been working the entire weekend after his passing to spread the news and collect photographs, “it is an honour to do this last task for him”. His first secretary from his time at KFO (Marijke Oskam) had stayed in contact for over 50 years and was at his farewell.
Former PhD student, Leah, writes about Ben: “Ben let me stay at his beautiful house on my first daunting night in the Netherlands. A couple of times he entertained all the non-Dutch students at his house with fine food & wine, making everyone welcome. I best remember Ben for the intellectual debates because we were creating something new, trying to push frontiers and share our results with the world. Hierarchy, age and experience didn't matter in an argument, only finding the truth. Ben encouraged us all to correct each other, even during ESTRO talks, and that fostered a healthy, productive research environment. Ben was also amazingly positive when needed, before a scary talk, when the results from last night's measurements till late didn't make sense and when referees just didn't get the point.”

Fig. 11: Ben Mijnheer multi-tasking at ESTRO 2009
Indeed since 2005, with the culmination of Ben’s dedication and infectious enthusiasm, almost all IMRT and VMAT patient treatments at NKI have been validated with EPID dosimetry and important faults have been prevented for a small fraction of treatments. I think it is fair to say that Ben Mijnheer is the father of in vivo EPID dosimetry. Igor Olaciregui-Ruiz once asked, ‘Ben, how come that you keep working so tirelessly years after retirement?’ You responded: ’How can you ask a painter to stop painting’ (Fig. 11).
Ben is survived by his two daughters and their mother, as well as by his wife. We wish them strength in these difficult times and thank them for their endless support that has helped Ben achieve the abovementioned things. He remains an inspiration for researchers and clinical scientists in radiotherapy and he shall be sorely missed.
Acknowledgements
I would like to thank Henk van der Gugten and Pat Fewer for help in reconstructing Ben’s timeline and Leah McDermott, Harry Bartelink and Els Couenberg, Anton Mans, Jan-Jakob Sonke and Mariia Gavrylenko-Mijnheer for proofreading this article.
References
[1] McDermott LN, Wendling M, van Asselen B, Stroom J, Sonke JJ, van Herk M, Mijnheer BJ. Clinical experience with EPID dosimetry for prostate IMRT pre-treatment dose verification. Med Phys. 2006 Oct;33(10):3921-30. doi: 10.1118/1.2230810. PMID: 17089854.
[2] Mans A, Wendling M, McDermott LN, Sonke JJ, Tielenburg R, Vijlbrief R, Mijnheer B, van Herk M, Stroom JC. Catching errors with in vivo EPID dosimetry. Med Phys. 2010 Jun;37(6):2638-44. doi: 10.1118/1.3397807. PMID: 20632575.
[3] Dogan N, Mijnheer BJ, Padgett K, Nalichowski A, Wu C, Nyflot MJ, Olch AJ, Papanikolaou N, Shi J, Holmes SM, Moran J, Greer PB. AAPM Task Group Report 307: Use of EPIDs for Patient-Specific IMRT and VMAT QA. Med Phys. 2023 Aug;50(8):e865-e903. doi: 10.1002/mp.16536. Epub 2023 Jun 29. PMID: 37384416.
[4] Mijnheer B, Ed., Clinical 3D Dosimetry in Modern Radiation Therapy. CRC press, ISBN 9780367870478, 2018.
[5] Mijnheer BJ, Aten AH Jr. Determination of fast neutron flux densities by the activation of phosphorus compounds. Health Phys. 1966 Aug;12(8):1142-3. PMID: 5968660.
[6] Mijnheer B, Jornet N. Interview with Ben Mijnheer, Eur. Medical Physics News 2013.
[7] Broerse JJ, Broers-Challiss JE, Mijnheer BJ. Depth-dose measurements of d-T neutrons for radiotherapy applications. Strahlentherapie. 1975 Jun;149(6):585-96. PMID: 1188992.
[8] Zoetelief J, Engels AC, Broerse JJ, Mijnheer BJ. Effect of finite size of ion chambers used for neutron dosimetry. Phys Med Biol. 1980 Nov;25(6):1121-31. doi: 10.1088/0031-9155/25/6/010. PMID: 7208625.
[9] Mijnheer BJ, Wieberdink T, Battermann JJ. In vivo dosimetry in the pelvis during fast neutron therapy. Br J Radiol. 1981 Jan;54(637):56-60. doi:10.1259/0007-1285-54-637-56. PMID: 7448502.
[10] Mijnheer BJ, Battermann JJ, van der Laarse R. Treatment planning at the Amsterdam d + T fast neutron therapy facility. Strahlentherapie Sonderb. 1981;77:102-10. PMID: 6820982
[11] Williams JR, Mijnheer BJ, Rassow J, Meissner P, Hensley F. A small-scale neutron dosimetry intercomparison between Essen, Amsterdam and Edinburgh. Strahlentherapie. 1981 Apr;157(4):245-50. PMID: 7245265.
[12] Broerse JJ, Mijnheer BJ, Williams JR. European protocol for neutron dosimetry for external beam therapy. European Clinical Neutron Dosimetry Group (ECNEU). Br J Radiol. 1981 Oct;54(646):882-98. doi:10.1259/0007-1285-54-646-882. PMID: 6794701.
[13] Battermann JJ, Mijnheer BJ. The Amsterdam fast neutron therapy project: a final report. Int J Radiat Oncol Biol Phys. 1986 Dec;12(12):2093-9. doi:10.1016/0360-3016(86)90007-6. PMID: 3793545.
[14] Konijnenberg MW, Mijnheer BJ, Raaijmakers CP, Stecher-Rasmussen F, Watkins PR. An investigation of the possibilities of BNCT treatment planning with the Monte Carlo method. Strahlenther Onkol. 1993 Jan;169(1):25-8. PMID: 8434336.
[15] Mijnheer BJ, Battermann JJ, Wambersie A. What degree of accuracy is required and can be achieved in photon and neutron therapy? Radiother Oncol. 1987 Mar;8(3):237-52. doi: 10.1016/s0167-8140(87)80247-5. PMID: 3107087.
[16] van der Merwe D, Van Dyk J, Healy B, Zubizarreta E, Izewska J, Mijnheer B, Meghzifene A. Accuracy requirements and uncertainties in radiotherapy: a report of the International Atomic Energy Agency. Acta Oncol. 2017 Jan;56(1):1-6. doi: 10.1080/0284186X.2016.1246801. Epub 2016 Nov 16. PMID: 27846757.
[17] van der Laarse R, Strackee J. Pseudo optimization of radiotherapy treatment planning. Br J Radiol. 1976 May;49(581):450-7. doi: 10.1259/0007-1285-49-581-450. PMID: 949579.
[18] Bruinvis IA, Van Amstel A, Elevelt AJ, Van der Laarse R. Calculation of electron beam dose distributions for arbitrarily shaped fields. Phys Med Biol. 1983 Jun;28(6):667-83. doi: 10.1088/0031-9155/28/6/007. PMID: 6410420.
[19] Meertens H, van Herk M, Weeda J. A liquid ionisation detector for digital radiography of therapeutic megavoltage photon beams. Phys Med Biol. 1985 Apr;30(4):313-21. doi: 10.1088/0031-9155/30/4/004. PMID: 3923505.
[20] van Herk M, Meertens H. A matrix ionisation chamber imaging device for on-line patient setup verification during radiotherapy. Radiother Oncol. 1988 Apr;11(4):369-78. doi: 10.1016/0167-8140(88)90208-3. PMID: 3131843.
[21] Petti PL, Rice RK, Mijnheer BJ, Chin LM, Bjärngard BE. Heterogeneity model for photon beams incorporating electron transport. Med Phys. 1987 May-Jun;14(3):349-54. doi: 10.1118/1.596048. PMID: 3600523.
[22] Mijnheer BJ, Chin LM. The effect of differences in data base on the determination of absorbed dose in high-energy photon beams using the American Association of Physicists in Medicine protocol. Med Phys. 1989 Jan-Feb;16(1):119-22. doi: 10.1118/1.596398. PMID: 2921969.
[23] Obcemea CH, Rice RK, Mijnheer BJ, Siddon RL, Tarbell NJ, Mauch P, Chin LM. Three-dimensional dose distribution of total body irradiation by a dual source total body irradiator. Int J Radiat Oncol Biol Phys. 1992;24(4):789-93. doi:10.1016/0360-3016(92)90730-6. PMID: 1429106.
[24] Heukelom S, Lanson JH, Mijnheer BJ. Comparison of entrance and exit dose measurements using ionization chambers and silicon diodes. Phys Med Biol. 1991 Jan;36(1):47-59. doi: 10.1088/0031-9155/36/1/005. PMID: 2006214.
[25] Essers M, Mijnheer BJ. In vivo dosimetry during external photon beam radiotherapy. Int J Radiat Oncol Biol Phys. 1999 Jan 15;43(2):245-59. doi: 10.1016/s0360-3016(98)00341-1. PMID: 10030247.
[26] Essers M, Lanson JH, Leunens G, Schnabel T, Mijnheer BJ. The accuracy of CT-based inhomogeneity corrections and in vivo dosimetry for the treatment of lung cancer. Radiother Oncol. 1995 Dec;37(3):199-208. doi: 10.1016/0167-8140(95)01659-7. PMID: 8746588.
[27] Boellaard R, van Herk M, Mijnheer BJ. The dose response relationship of a liquid-filled electronic portal imaging device. Med Phys. 1996 Sep;23(9):1601-11. doi: 10.1118/1.597828. PMID: 8892258.
[28] Essers M, Boellaard R, van Herk M, Lanson H, Mijnheer B. Transmission dosimetry with a liquid-filled electronic portal imaging device. Int J Radiat Oncol Biol Phys. 1996 Mar 1;34(4):931-41. doi: 10.1016/0360-3016(95)02191-4. PMID: 8598373.
[29] Boellaard R, Essers M, van Herk M, Mijnheer BJ. New method to obtain the midplane dose using portal in vivo dosimetry. Int J Radiat Oncol Biol Phys. 1998 May 1;41(2):465-74. doi: 10.1016/s0360-3016(98)00048-0. PMID: 9607366.
[30] Louwe RJ, Damen EM, van Herk M, Minken AW, Törzsök O, Mijnheer BJ. Three-dimensional dose reconstruction of breast cancer treatment using portal imaging. Med Phys. 2003 Sep;30(9):2376-89. doi: 10.1118/1.1589496. PMID: 14528960.
[31] McDermott LN, Louwe RJ, Sonke JJ, van Herk MB, Mijnheer BJ. Dose-response and ghosting effects of an amorphous silicon electronic portal imaging device. Med Phys. 2004 Feb;31(2):285-95. doi: 10.1118/1.1637969. PMID: 15000614.
[32] Wendling M, McDermott LN, Mans A, Sonke JJ, van Herk M, Mijnheer BJ. A simple backprojection algorithm for 3D in vivo EPID dosimetry of IMRT treatments. Med Phys. 2009 Jul;36(7):3310-21. doi: 10.1118/1.3148482. PMID: 19673227.
[33] Rozendaal RA, Mijnheer BJ, Hamming-Vrieze O, Mans A, van Herk M. Impact of daily anatomical changes on EPID-based in vivo dosimetry of VMAT treatments of head-and-neck cancer. Radiother Oncol. 2015 Jul;116(1):70-4. doi: 10.1016/j.radonc.2015.05.020. Epub 2015 Jun 30. PMID: 26142267.
[34] Spreeuw H, Rozendaal R, Olaciregui-Ruiz I, González P, Mans A, Mijnheer B, van Herk M. Online 3D EPID-based dose verification: Proof of concept. Med Phys. 2016 Jul;43(7):3969. doi: 10.1118/1.4952729. PMID: 27370115.
[35] Torres-Xirau I, Olaciregui-Ruiz I, Baldvinsson G, Mijnheer BJ, van der Heide UA, Mans A. Characterization of the a-Si EPID in the unity MR-linac for dosimetric applications. Phys Med Biol. 2018 Jan 9;63(2):025006. doi:10.1088/1361-6560/aa9dbf. PMID: 29182153.
[36] Olaciregui-Ruiz I, Beddar S, Greer P, Jornet N, McCurdy B, Paiva-Fonseca G, Mijnheer B, Verhaegen F. In vivo dosimetry in external beam photon radiotherapy: Requirements and future directions for research, development, and clinical practice. Phys Imaging Radiat Oncol. 2020 Aug 29;15:108-116. doi: 10.1016/j.phro.2020.08.003. PMID: 33458335; PMCID: PMC7807612.
[37] Bos LJ, Damen EM, de Boer RW, Mijnheer BJ, McShan DL, Fraass BA, Kessler ML, Lebesque JV. Reduction of rectal dose by integration of the boost in the large-field treatment plan for prostate irradiation. Int J Radiat Oncol Biol Phys.2002 Jan 1;52(1):254-65. doi: 10.1016/s0360-3016(01)02676-1. PMID: 11777644.
[38] Claus F, Mijnheer B, Rasch C, Bortfeld T, Fraass B, De Gersem W, Wirtz H, Hoinkis C, Cho BC, Kwong LW, Bae H, Muller K, De Neve W. Report of a study on IMRT planning strategies for ethmoid sinus cancer. Strahlenther Onkol. 2002 Oct;178(10):572-6. doi: 10.1007/s00066-002-0999-3. PMID: 12386789.
[39] Damen EM, Brugmans MJ, van der Horst A, Bos L, Lebesque JV, Mijnheer BJ, McShan DL, Fraass BA, Kessler ML. Planning, computer optimization, and dosimetric verification of a segmented irradiation technique for prostate cancer. Int J Radiat Oncol Biol Phys. 2001 Mar 15;49(4):1183-95. doi: 10.1016/s0360-3016(00)01525-x. PMID: 11240262.
[40] Schwarz M, Alber M, Lebesque JV, Mijnheer BJ, Damen EM. Dose heterogeneity in the target volume and intensity-modulated radiotherapy to escalate the dose in the treatment of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2005 Jun 1;62(2):561-70. doi: 10.1016/j.ijrobp.2005.02.011. PMID: 15890601.
[41] Cho BC, Mijnheer BJ, Bartelink H. Determining optimal two-beam axial orientations for heart sparing in left-sided breast cancer patients. Med Phys.2004 Jan;31(1):111-21. doi: 10.1118/1.1634391. PMID: 14761027.
[42] Lamers-Kuijper E, Schwarz M, Rasch C, Mijnheer B. Intensity-modulated vs. conformal radiotherapy of parotid gland tumors: potential impact on hearing loss. Med Dosim. 2007 Winter;32(4):237-45. doi: 10.1016/j.meddos.2006.12.004. PMID: 17980823.
[43] Steggerda MJ, Mijnheer BJ. Replacement corrections of a Farmer-type ionization chamber for the calibration of Cs-137 and Ir-192 sources in a solid phantom. Radiother Oncol. 1994 Apr;31(1):76-84. doi: 10.1016/0167-8140(94)90415-4. PMID: 8041900.
[44] van Elmpt W, Nijsten S, Petit S, Mijnheer B, Lambin P, Dekker A. 3D in vivo dosimetry using megavoltage cone-beam CT and EPID dosimetry. Int J Radiat Oncol Biol Phys. 2009 Apr 1;73(5):1580-7. doi: 10.1016/j.ijrobp.2008.11.051. PMID:19306755.
[45] Mijnheer BJ, Wittkämper FW. Comparison of recent codes of practice for high-energy photon dosimetry. Phys Med Biol. 1986 Apr;31(4):407-16. doi: 10.1088/0031-9155/31/4/006. PMID: 3090570.
[46] Georg D, Heukelom S, Venselaar J. Formalisms for MU calculations, ESTRO booklet 3 versus NCS report 12. Radiother Oncol. 2001 Sep;60(3):319-28. doi: 10.1016/s0167-8140(01)00348-6. PMID: 11514012.
[47] Dutreix AD, Bjärngard BE, Bridier A, Mijnheer B, Shaw JE, Svensson H (1997) Monitor unit calculation for high energy photon beams. Physics for Clinical Radiotherapy. Booklet No. 3, pp. 1-151. Garant, Leuven, Belgium
[48] Mijnheer B, Bridier A, Garibaldi C, Torzsok K, Venselaar J (2001) Monitor Unit Calculation For High-Energy Photon Beams – Practical Examples ESTRO Booklet No. 6 European Society for Therapeutic Radiology and Oncology, Brussels, Belgium
[49] Mijnheer B, Olszewska A, Fiorino C, Hartmann G, Knöös T, Rosenwald J-C, Welleweerd H (2004) Quality assurance of treatment planning systems - practical examples for non-IMRT photon beams ESTRO Booklet No. 7 European Society for Therapeutic Radiology and Oncology, Brussels, Belgium
[50] Alber M, Broggi S, De Wagter C, Eichwurzel I, Engstrom P, Fiorino C, Georg D, Hartmann G, Knoos, T, Leal A, Marijnissen H, Mijnheer B, Paiusco M, Sanchez-Doblado F, Schmidt R, Tomse M, Welleweerd H (2008) Guidelines for verification of IMRT. ESTRO Booklet No. 9 European Society for Therapeutic Radiology and Oncology, Brussels, Belgium
[51] Korreman S, Rasch C, McNair H, Verellen D, Oelfke U, Maingon P, Mijnheer B, Khoo V. The European Society of Therapeutic Radiology and Oncology-European Institute of Radiotherapy (ESTRO-EIR) report on 3D CT-based in-room image guidance systems: a practical and technical review and guide. Radiother Oncol. 2010 Feb;94(2):129-44. doi: 10.1016/j.radonc.2010.01.004. Epub 2010 Feb 12. PMID: 20153908.
[52] Reft C, Alecu R, Das IJ, Gerbi BJ, Keall P, Lief E, Mijnheer BJ, Papanikolaou N, Sibata C, Van Dyk J; AAPM Radiation Therapy Committee Task Group 63. Dosimetric considerations for patients with HIP prostheses undergoing pelvic irradiation. Report of the AAPM Radiation Therapy Committee Task Group 63. Med Phys. 2003 Jun;30(6):1162-82. doi: 10.1118/1.1565113. PMID: 12852541.
[53] McKenzie A, van Herk M, Mijnheer B. Margins for geometric uncertainty around organs at risk in radiotherapy. Radiother Oncol. 2002 Mar;62(3):299-307. doi: 10.1016/s0167-8140(02)00015-4. PMID: 12175561.